-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2012; 1(4): 47-51
doi: 10.5923/j.diabetes.20120104.01
Assessment of Antidiabetic Potential of Ficus Sycomorus on Alloxan-induced Diabetic Mice
Njagi JM 1, Piero MN 1, Ngeranwa JJN 2, Njagi ENM 2, Kibiti CM 3, Njue WM 4, Maina D 5, Gathumbi PK 6
1Department of Environmental Health, Kenyatta University, P.O Box 43844-00100, Nairobi, Kenya
2Department of Biochemistry and Biotechnology, Kenyatta University, P.O Box 43844-00100, Nairobi, Kenya
3Department of Pure and Applied Sciences, Mombasa Polytechnic University College, P.O Box 90420-80100, Mombasa, Kenya
4Department of Chemistry, Kenyatta University, P.O. Box 43844-00100 Nairobi, Kenya
5Institute of Nuclear Science, University of Nairobi, P.O Box 30197-00200 Nairobi, Kenya
6Department of Veterinary Pathology, Microbiology and Parasitology, University of Nairobi, P.O. Box 29053-00625 Nairobi, Kenya
Correspondence to: Njagi JM , Department of Environmental Health, Kenyatta University, P.O Box 43844-00100, Nairobi, Kenya.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Diabetes mellitus is a predominant public health concern, causing substantial morbidity, mortality, and long-term complications. Many of the conventional drugs used for the management of this disease are not only expensive but also have numerous side effects. Herbal medications are cheaper and locally available. Many plants have been traditionally used to manage diabetes without authentication on their antidiabetic properties and assessment of their safety. In this study aqueous stem bark extract of Ficus sycomorus was assessed for its antidiabetic potential along with evaluation its preliminary in vivo toxicity in alloxan-induced diabetic mice. Results show that the plant harbours remarkable antidiabetic potential. It safely lowered blood glucose level to levels below what insulin, the model drug, lowers, in a dose-dependent manner.
Keywords: Diabetes Mellitus, Ficus Sycomorus, Preliminary in Vivo Toxicity, Herbal Medications
Cite this paper: Njagi JM , Piero MN , Ngeranwa JJN , Njagi ENM , Kibiti CM , Njue WM , Maina D , Gathumbi PK , "Assessment of Antidiabetic Potential of Ficus Sycomorus on Alloxan-induced Diabetic Mice", International Journal of Diabetes Research, Vol. 1 No. 4, 2012, pp. 47-51. doi: 10.5923/j.diabetes.20120104.01.
Article Outline
1. Introduction
- Diabetes mellitus is a metabolic disorder affecting the body’s ability to make or utilize insulin, a pancreatic hormone that helps transport glucose (blood sugar) from the bloodstream into the cells so they can break it down and use it for fuel. The disease results in abnormal levels of glucose in the bloodstream. This can cause severe short-term and long-term consequences ranging from brain damage to amputations and heart disease[1]. Diabetes mellitus has an estimated global prevalence of 4.4% by the year 2030. The number of diabetics worldwide is expected to rise from 171 million in 2000 to 366 million in 2030[2]. The clinical diagnosis of diabetes is often indicated by the presence of symptoms such as polyuria, polydipsia, and unexplained weight loss, and is confirmed by measurement of abnormal hyperglycaemia[3].Recently, the incidence of diabetes has soared worldwide and is expected to keep growing, with the greatest increase seen in type II diabetes mellitus. This is largely attributed to the rise of obesity and the global spread of Western-style habits viz; physical inactivity along with high caloric diets, processed carbohydrates and saturated fats and insufficient in fiber-rich whole foods. The aging of the population is also a predisposing factor. However, other factors, such as environment may also be contributing, because cases of autoimmune diabetes (type I) are also becoming morecommon[4]. The estimated number of people with diabetes has jumped from 30 million in 1985 to 150 million in 2000 and then to 246 million in 2007, according to the International Diabetes Federation[4]. Diabetes mellitus is conventionally managed by insulin injection, oral hypoglycemic agents, exercise, acupuncture, proper diet[5]. In developing world, traditional herbal medicines have continued to play an important role side by side with modern medicine especially in primary health care in poorer or rural areas. Herbal preparations constitute valuable natural resources from which chemicals of great potential for agriculture and medicine are found[6].Plant derivatives with presumed hypoglycemic properties have been used in folk medicine and traditional healing systems globally such as Native American Indian, Jewish Chinese East Indian and Mexican[7]. Many modern pharmaceuticals used in conventional medicine have also natural plant origins. Among them metformin, derived from the flowering plant, Galega officinalis, is a common traditional remedy for diabetes[8,9].It is against this background that this study was undertaken to evaluate the hypoglycemic potential of aqueous stem bark extract of Ficus sycomorus, a Kenyan medicinal plant. Its preliminary in vivo toxicity was also assessed. It is used by local herbalists to ameliorate diabetes complications. However, its antidiabetic potential in vivo safety had not been authenticated.
2. Materials and Methods
2.1. Collection of Plant Materials
- Stem barks of the medicinal plant Ficus sycomorus were collected from their natural habitat in Makunguru village in Siakago division of Mbeere North district in Embu county, Kenya, on the basis of the folklore reports from local practicing herbalists on hypoglycemic activity of the plant. An acknowledged authority in taxonomy identified the plant and voucher specimen was deposited at the National Museum of Kenya herbarium. The voucher specimen number of the plant was Hypo 1/06. The stem barks were cut into small pieces and air-dried at 25±3℃ away from direct sunlight for three weeks. The dried materials were then crushed into powder by use of an electric mill (Christy and Norris Ltd, England).
2.2. Preparation of Extracts
- The aqueous stem bark extracts of F. sycomorus were prepared by boiling 100g of crushed material in one liter of distilled water for two hours with frequent stirring. The mixture was left to cool at room temperature and then decanted into a dry clean conical flask through folded cotton gauze stuffed into a funnel. Following decantation, the extract was filtered using Whatmann no. 1 filter papers by use of a vacuum pump. The filtrate was freeze-dried for 72 hours. Afterwards the powder stored at 4℃ in airtight containers.
2.3. Extract Dose Formulation
- Physiological saline was prepared by dissolving 0.85g of analytical grade NaCl in 100 ml of distilled water. The extract for injection was prepared as follows: the 50mg/kg body weight dose was prepared by dissolving 12.5mg in 1ml of physiological saline; the 100mg/kg body weight dose was prepared by dissolving 25mg in 1ml of physiological saline; and the 150mg/kg body weight dose was prepared by dissolving 37.5mg in 1ml of physiological saline. The experimental animals were given 0.1 ml of the extract solutions. Insulin was also reconstituted and animals were given 0.1 ml.
2.4. Hypoglycemic Bioscreening
2.4.1. Laboratory Animals
- 4-6 weeks old healthy Swiss albino male mice weighing 23-27 g were used for the study. They were fed on the standard mice pellet diet and allowed free access to water ad libitum. Permission for use of laboratory animals in this study was sought and granted by animal rights agency in Nairobi, Kenya. For experimental purposes, the animals were fasted overnight and allowed free access to water. The animals were divided into 4 groups of four animals each. Group 1 (Non-diabetic mice) was given 0.1ml of normal saline; Group 2, (diabetic mice) was given 0.1ml of normal saline; Group 3, (diabetic mice) was given insulin at a dose of 1 IU/kg body weight; and Group 4, (diabetic mice) was given plant extracts at three dose levels (50mg/kg body weight, 100mg/kg body weight and 150mg/kg body weight). Each dose level was given to 4 animals. Group 1 and 2 served as controls whereas Group 3 served as the reference group. Diabetic condition was induced in mice byintraperitoneal injection of alloxan monohydrate (150mg/kgbw) [sourced from Fluka chemie Gmbhch 9471 Switzerland] 72 hours before the start of experiment. Before administration of the different treatments the animals were bled and blood glucose level in the animals was measured. This was the initial measurement at time zero. The animals were again bled hourly until the fourth hour.
2.4.2. Blood Sample Collection
- Blood samples were collected from the tails of the animals after wiping the tail with surgical spirit. The tail was nibbed by use of a pair of sharp scissors, a drop of blood was squeezed into a Glucometer. After collection of blood, the nibbled side of the tail was rubbed with cotton wool soaked in absolute ethanol to protect the animal from infection and to arrest further bleeding.
2.4.3. Blood Glucose Level Determination
- The principle of the test is based on a glucoseoxidase/peroxidase reaction, which is specific for β-D-glucose. The hypoguard machine was used together with GB Supreme blood glucose test strips. The Supreme Test Strip is a disposable plastic strip containing a chemically treated test area used to measure the amount of blood glucose. The test area is designed in such a way that when a drop of blood is placed on the top surface, color change occurs which is determined by a Supreme Hypoguard meter. The supreme Test Strip was fully inserted into the meter before applying a drop of blood to fully cover the test area inside the grey target. The test strips and the Supreme Hypoguard Glucometer were obtained from Hypoguard Ltd, United Kingdom through Chemoquip Ltd, Kenya.
2.5. Preliminary in Vivo Toxicity Analysis
- Ten laboratory animals (Male Swiss albino mice) were used for preliminary in vivo toxicity analysis. Five animals were treated with saline and served as controls. The other five animals were treated with 450 mg /kg body weight of the aqueous stem bark extract of F. sycomorus. The animals were injected with the extracts daily for 30 days and kept under close observation and fed on standard mice pellets and tap water ad libitum. Any animal that died or showed signs of death was sacrificed. The animals that were still alive after 30 days were sacrificed. The animals were dissected and pieces of pancreas, heart, kidney, muscle and livers were removed and preserved in 10 % formalin for histological preparation and observation. The tissues were processed for histopathological examination under a microscope for any pathological changes.
2.6. Data Analysis
- Collected data on hypoglycemic activity bioscreening was analysed by unpaired student’s t-test (Instat statistical software) to evaluate the significance between means of extract treated animals and the diabetic control, insulin control and the normal control. The data was represented as means ± SEM. P<0.05 was considered statistically significant.
3. Results
3.1. Effect of Ficus Sycomorus on Blood Glucose in Alloxan-Induced Diabetic Mice
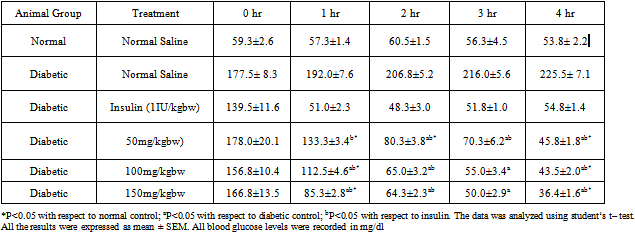
- The percent reductions of blood glucose levels in mice by the aqueous stem bark extract of Ficus sycomorus at the three dose levels (50 mg/kg body weight, 100mg/kg body weight and 150 mg/kg body weight) during the 1st hour was 30%, 28 % and 49%, respectively. At this hour the plant extract lowered the blood glucose levels but not to normal (*P<0.05)(Figure 1). The doses, however, significantly lowered blood glucose levels as compared to the diabetic control (aP<0.05) (Table 1). During the 2nd hour the glucose lowering effect by the three dose levels was also observed, as the percentage reduction of blood glucose was 54%, 58% and 61% respectively.
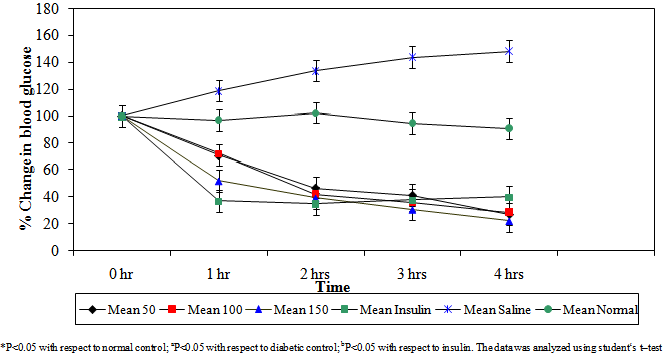 | Figure 1. Percentage reduction in blood glucose by varying doses of Ficus sycomorus in diabetic mice |
|
- The extract lowered blood glucose levels to normal (bP>0.05). In the 3rd hour the extract lowered blood glucose levels by 59%, 65% and 70% respectively. At this hour the extract lowered blood glucose levels as effectively as insulin (bP<0.05) especially by the 150 mg/kg body weight dose range. The same trend was observed during the 4th hour where the three dose levels lowered blood glucose levels lower than even insulin. The percentage blood glucose reductions were 72%, 73% and 78% respectively (bP<0.05). The aqueous stem bark extract of Ficus sycomorus caused a steady decrease in blood glucose levels in the diabetic control mice during the 1st and 2nd hours and then a steep decrease during the 3rd and 4th hours for all the doses (Table 1).
3.2. Preliminary In Vivo Toxicity
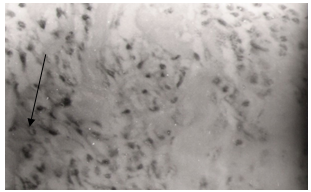
- The aqueous stem bark extract of Ficus sycomorus caused local irritation to the body tissues at the point of injection. This was evident after histopathological examination of skeletal muscle tissue near the point of injection, which demonstrated by pyrogranulomatous inflammation of the soft tissue. The kidney cells were not damaged but there was mild perirenal steatite (Figure 2). Liver, spleen and heart tissues were normal as there were no signs of pathology.
4. Discussion
- The aqueous stem bark extracts of Ficus sycomorus showed potent hypoglycemic activity in alloxan-induced diabetic mice. This observation was similar made by[10], who demonstrated remarkable hypoglycemic activity on streptozotocin induced diabetic mice on administration of aqueous leave extracts of Camellia sinensis. The fact that the aqueous stem extract of Ficus sycomorus demonstrated a dose dependent response on blood glucose lowering effect on alloxan induced diabetic mice is consistent with the observation made by[11] in studies carried out on streptozotocin induced diabetic rats orally administered with leave extracts of Eucalyptus globulus. In another related study[12] demonstrated a dose dependent hypoglycemic activity on alloxan-induced diabetic rabbits onadministration of fresh leave extracts of Catharanthus roseus Linn. Further, it was similarly observed by[13], whodemonstrated hypoglycemic activity on normal and alloxan-induced diabetic rats intraperitoneally injected with methanolic extracts of Ocimum gratissimum leaves. This indicates that this plant extract might have been absorbed through the cell lipids membrane through facilitated diffusion. The ions might have been transported in the direction of their electrochemical gradient. This trend is in agreement with expectations seen in administration of higher concentration of drug. The plant extract also lowered blood glucose level to levels below what insulin, the model drug, lowers (Figure 1 and Table 1). This suggests the possibility of islet repair or mimicry of insulin action by elements from the extract.The range of doses used in this study were within the doses used by[10] and[11].[10],while examining the hypoglycemic effect of green tea on blood glucose levels, used a dose range of 30-300mg/kg body weights in rats.[11] used a dose range of 150 and 300 mg/kg body weight while evaluating the hypoglycemic activity of aqueous extract of Eucalyptus globulus in normal and streptozotocin-induced diabetic rats.[11] used a dose of 4.5 g/kg body weight of Eucalyptus globulus leaf extract to evaluate toxicity of this plant extract. The aqueous stem bark extract F. sycomorus did not alter the normal cell structure of the heart, kidney, liver and spleen. This perhaps suggests the safety of this plant when used to traditionally manage diabetes mellitus. The inflammation at the site of injection may also be attributed to drug induced reactions[14]. The surface irritations on the kidney that were present in the plant extract may suggest that it contains phytochemical compounds such as tannins and saponins, which are known to cause such effects (Diwan, 2000). This is an indication that phytochemical screening of the plant extract is necessary.
5. Conclusions
- The aqueous stem bark extract of Ficus sycomorus showed significant antidiabetic potential. The results of this study, therefore, confirm its suitability for the management of diabetes mellitus. However, its mode of antidiabetic action is still obscure. Histological responses induced by the extracts indicated no overt pathology attributable to this plant afterinterperitoneal administration. This might indicate its low acute toxicity after interperitoneal administration. Further toxicity tests using the oral route of administration at standardized dosage levels will be needed to comprehensively evaluate the safety of this plant extract in animal models after administration through the conventionally used route of administration for the management of diabetes mellitus.Further studies on this plant is are needed using organic solvents followed by solation of pure antidiabetic compounds. This will invoke desire for design of newer and more efficacious glucose-lowering drugs. The study was, nevertheless, justified.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the following persons and institutions for their immense contribution in execution of this study; The late Mr. Solomon Buleti and Mr. Patrick Muiruri for assistance in laboratory animal handling, Rose Gitari and Jackson Gachoka for assistance in preliminary in vivo toxicity analysis, Mr. Mugeki, a traditional herbal practitioner, for assistance in collection of the medicinal plant and Kenyatta University and University of Nairobi for providing facilities where this work was undertaken.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML