-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2012; 1(3): 42-46
doi: 10.5923/j.diabetes.20120103.03
Lack of Association of the Ghrelin Gene Arg51Gln Single Nucleotide Polymorphism with Obesity and Metabolic Syndrome among Multi-ethnic Malaysian Subjects
Sher-Wyn Lui 1, Phee-Phee Chia 2, Yee-How Say 1
1Department of Biomedical Science, Faculty of Science
2Department of Science and Engineering, Centre for Foundation Studies Universiti Tunku Abdul Rahman (UTAR) Perak Campus, 31900 Kampar, Perak, Malaysia
Correspondence to: Yee-How Say , Department of Biomedical Science, Faculty of Science.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Obesity and metabolic syndrome has become a public health concern because of its association with a number of medical complications that lead to increased morbidity and mortality. Ghrelin is a hormone that is primarily secreted in the stomach, which plays an important role to increase hunger through its action on hypothalamic feeding. The Ghrelin gene Arg51Gln single nucleotide polymorphism (SNP) (rs34911341) has been associated with obesity and metabolic syndrome in previous studies. Therefore, this study was to examine the prevalence of this SNP and its association with obesity, obesity-related traits and metabolic syndrome among 184 multi-ethnic Malaysian subjects (67 males, 117 females; 76 obese, 108 non obese; 52 Malay, 91 ethnic Chinese, 41 ethnic Indians) from the Kampar Health Clinic cohort. Demographic data, anthropometric and clinical measurements of subjects were collected. Genotyping was performed by using the genomic DNA extracted from leukocytes, followed by Polymerase Chain Reaction and SacI Restriction Fragment Length Polymorphism, revealing 113 GG, 70 GA and 1 AA subjects; minor allele frequency 0.196. Arg51Gln alleles did not show any association with obesity (p = 0.643), gender (p = 0.064) and ethnicity (p = 0.390). Besides, it did not show any association with the presence of metabolic syndrome according to 3 criteria in the modified NCEP ATP III for Asians (p = 0.931). Anthropometric and clinical measurements indicative of obesity and metabolic syndrome were also all not significantly different between the alleles. In conclusion, the Ghrelin Arg51Gln gene variant was not associated with obesity, obesity-related traits and metabolic syndrome among Malaysian subjects in this study.
Keywords: Ghrelin, Single Nucleotide Polymorphism, Obesity, Metabolic Syndrome, Anthropometric Measurements, Malaysia
Article Outline
1. Introduction
- Obesity is a medical condition in which excess body fat has accumulated to the extent that it may have an adverse effect on health, leading to reduced life expectancy and increased health problems. It is a multifactorial disease caused by an interaction of genetic factors with lifestyle and environmental factors, and is rapidly increasing worldwide[1]. The 2006 Third Malaysian National Health and Morbidity Survey (NHMS III) found that the prevalence of overweight had increased to 29.1% and that of obese – 14.0%; compared to the 1996 NHMS II at 16.6% and 4.0%, respectively[2]. Obesity is a public health concern because its increased prevalence has been accompanied by a parallel increase in the prevalence of the metabolic syndrome (MetS) or “syndrome X”[3]. Metabolic syndrome is a heterogeneous disorder which is characterized by the presence of three or more of the criteria which include abdominal obesity, elevated triglyceride concentrations, low high-density lipoprotein (HDL) cholesterol, high blood pressure, and elevated fasting glucose[3]. Ghrelin is a synthesized as pre-pro-hormone, then proteolytically processed to yield a 28 amino acid peptide[4]. The human ghrelin gene (Ghrelin) is located on chromosome 3, at locus 3p25-26, and consists of 4 exons and 3 introns[5]. Ghrelin is primarily produced in the stomach, as well as by other tissues like intestines, pancreas, hypothalamus, placenta, kidney, gonads and pituitary gland[4]. Ghrelin is the first natural hormone to be identified in which the hydroxyl group of one of its serine residues is acylated with an n-octanoyl[4]. Acylated ghrelin was discovered as the endogenous ligand of the growth hormone secretagogue receptor type 1a (GHSR1a), which stimulates growth hormone release and regulates appetite[6]. Ghrelin circulates in the bloodstream and shows pulsatile secretion, with levels higher on fasting and lower levels after food intake[6]. It modulates gastric motility and acid secretion, inhibits gastric emptying, and affects insulin and gastrin secretion, indicating that ghrelin has oxiregenic effect coupled with control of energy expenditure, gastric motility and acid secretion[6]. The influence of ghrelin on both endocrine and exocrine pancreatic function and glucose metabolism suggests that it would play a major role in the endocrine abnormalities commonly present in obesity[7].Previous studies have provided contradictory findings on various single nucleotide polymorphisms (SNPs) in Ghrelin as to their association with obesity and obesity-related phenotypes[8]. One common SNP detected is Arg51Gln (rs34911341), resulting from a single base substitution G152A, with Gln replacing Arg at codon 28 of mature ghrelin[9]. The Arg51Gln mutation results in a change in the COOH-terminal processing site of the ghrelin peptide within its precursor protein from Proline-Arginine to Proline-Glutamine, resulting in the failure of the normal cleavage necessary to produce the 28-amino acid ghrelin[10]. A 94-amino-acid long pro-ghrelin peptide may still be produced, although its biological activity has not been assessed[10]. Several association studies on the Arg51Gln SNP conducted in different populations mostly revealed negative associations - for example the Italian population[11] and the German populations[12,13], where the allele frequencies of the SNP were similar between non-obese and obese subjects. Also, according to the study of Pöykkö et al. (2003), the Arg51Gln variant was associated with T2DM and elevated blood pressure in the middle-aged Finnish subjects; however, was not associated obesity phenotypes[14]. Currently, there is limited data and evidence on this association among the Malaysian population. As different populations show different associations in the existing research, the data on the association of this Ghrelin SNP with obesity in other populations cannot be used to extrapolate for the Malaysian population. Therefore, the objective of this study was to perform genotyping of the Ghrelin Arg51Gln SNP among Malaysian subjects from a health clinic in Kampar, Perak to determine the prevalence of the mutated genotypes and alleles, and to investigate if there was any association with obesity. Demographic characteristics, anthropometric measurements, blood pressures and fasting plasma glucose level were also determined to investigate whether there is any association of these obesity and metabolic syndrome-related traits with the Ghrelin Arg51Gln SNP.
2. Methodology
2.1. Study Participants, Questionnaire and Measurements
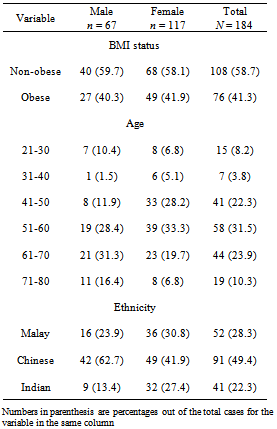
- A total of 184 unrelated subjects (age range: 21-80; overall mean age: 54.8 ± 13.6 – males: 57.8 8 ± 15.2, females: 53.2 ± 12.3) were recruited by convenience sampling from the Kampar Health Clinic from June to December, 2011, and their baseline characteristics are shown in Table 1. The exclusion criteria of the subjects include hyperthyroidism, pituitary diseases, chronic liver disease, chronic renal disease, acute infection, haematologic diseases and patients under medications that affect the glucose metabolism.
|
2.2. DNA Extraction and Genotyping
- Five millilitres of blood sample was collected and genomic DNA was then extracted from the nucleated leukocytes using the Wizard® Genomic DNA Purification Kit (Promega Inc., Madison, WI) as mentioned in our previous study[15]. Each of the PCR reaction vial contained 20 µl of solution, containing forward primer (2 mM), reverse primer (2 mM), PCR buffer with KCl (1×), Taq DNA polymerase recombinant (1 U/μl)(Fermentas, Lithuania), dNTP (0.2 mM) (Axygen Biosciences Inc., CA ) and MgCl2 (1.5 mM). The PCR was carried out using Biometra T Personal Thermocycler (Biometra GmbH, Germany), according to the conditions and primer sets used in a previous study[17]. The wild-type homozygous Arg51Arg refers to base G152G or known as the ‘GG’ genotype, heterozygous Arg51Gln refers to base G152A or ‘GA’ genotype, while mutated homozygous Gln51Gln refers to base A152A or ‘AA’ genotype. The fragments were resolved by 3 % agarose gel electrophoresis at constant 90 V for 45 min before staining with ethidium bromide and viewed under a UV transilluminator. The three genotypes were validated by sending to an outsourced DNA sequencing service.
2.3. Statistical Analysis
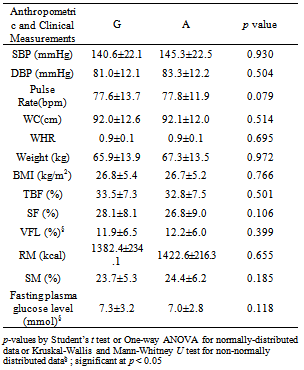
- Statistical Package for Social Students, IBM ®SPSS ® Statistics for Window® Version 16.0 (SPSS Inc., IL) was used to analyze the data. The normality of data was examined using One-Sample Kolmogorov-Smirnov Test whereby p > 0.05 indicates that the particular variable is normally distributed. Descriptive statistics was used to compute frequencies and percentages for demographic data, genotype and allele frequencies, and also to compute means and standard deviations for anthropometric measurements. Besides, Pearson’s Chi-square analysis was used to compare the difference in the genotype and allele distributions between groups. Means of clinical parameters were compared by Student’s t-test (between two variables) or using One-Way Analysis of Variance (ANOVA) (between more than two variables), except for VFL and fasting plasma glucose level whereby they were compared using Mann-Whitney U test (between two variables) or Kruskal-Wallis test (between more than two variables). In all statistical tests performed, p < 0.05 was denoted as statistically significant.
3. Results
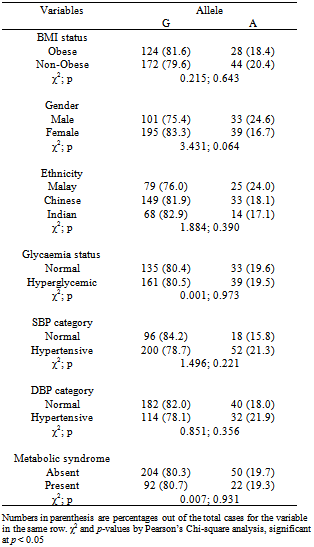
- The allele frequency for the mutated 51Gln or A allele (or known as Minor Allele Frequency, MAF) was 0.196, where 48 or 63.2% of the obese subjects carried GG genotype, 28 or 36.8% had GA genotype, while none had the AA genotype. The only AA genotype was detected in a normoglycaemic non-obese but hypertensive (based on SBP and DBP) Chinese male subject, aged 73. As there was only one subject with the AA genotype, we categorised the demographic and clinical variables based on alleles (Table 2).
|
|
4. Discussion
- The present study has demonstrated the existence of SNPs in the Ghrelin gene. One of the SNP reported in other studies[9,11,12-14], i.e. Arg51Gln, was also found in our study population, although without any association with obesity and metabolic syndrome. In our multi-ethnic subjects, the frequency for the 51Gln allele of 0.196 was much higher than the Swedish (0.031)[9], Italian (0.006)[11], German (0.016)[12] and Finnish (0.022)[14] Caucasian populations and Old Order Amish population (0.030)[18]. This rare Ghrelin Arg51Gln SNP was not even detected in two Chinese cohorts studied[17,19]. The Swedish study found association of this SNP with obesity[9], whereas the latter populations mentioned above did not. All these probably reflect genetic or ethnic heterogeneity between populations; therefore the data on the association of the Ghrelin Arg51Gln SNP with obesity in other populations cannot be used to extrapolate for the Malaysian population. Subjects with higher BMI tend to have low ghrelin concentration[20]. A previous study has found that the subjects of 51Gln allele had lower ghrelin concentrations[17]. This is due to the amino acid sequence of the mature peptide was modified by Arg51Gln, subsequently; the production of ghrelin level was reduced[17]. 51Gln carriers also had lower concentrations of insulin-like growth factor-1 (IGF-I) and higher concentrations of insulin-like growth factor binding protein 1 (IGFBP-1) compared to the non-carriers[21]. The reduced level of ghrelin concentrations were independently associated with several complications such as type 2 diabetes, insulin concentration, and insulin resistance and others[21]. Therefore, the Arg51Gln SNP has also been associated with components of metabolic syndrome, such as hyperglycaemia/type 2 diabetes and hypertension. According to the study of Xie et al. (2008), hyperglycaemia was found to be associated with the Arg51Gln SNP[22]. Other studies such as Pöykkö et al. (2008) and Krzyzanowska-Swiniarska et al. (2005) also reported that 51Gln allele carriers had higher prevalence of hypertension[21,23]. Therefore, the allele 51Gln was known a risk factor to hypertension, as low ghrelin level was found to be inversely associated to SBP and DBP[21,23]. However, there was absence of association of Ghrelin Arg51Gln SNP with hyperglycaemia, hypertension and metabolic syndrome in our study, again indicating genetic/ethnic heterogeneity between different populations.This study had some limitations whereby the respondents may not represent the whole Kampar population as only 184 subjects were studied. In addition, small sample size leads to the inconsistency of the results therefore limiting the power for statistical analysis and extrapolation. In future, the sample size of subjects should be increased. Younger adults will be more preferable as study subjects as the majority of the subjects that fell in the older age group of 51 to 60 in the current study may confound the results. In addition, lipid profiles such as triglyceride concentration and high-density lipoprotein cholesterol level could be included as well to expand the criteria for the diagnosis of metabolic syndrome. It will be interesting also to study the gene-environment interaction involved in obesity and metabolic syndrome, as dietary habits (such as high fat and high cholesterol diet) and lifestyle factors (such as physical activity) may affect Ghrelin gene activation and responses.
5. Conclusions
- In our study, we confirmed and replicated the findings of the German, Italian, Finnish, Old Order Amish and Chinese population studies for the non-association of Ghrelin Arg51Gln SNP with obesity, obesity-related traits and metabolic syndrome in this multi-ethnic Malaysian study group. The distribution of the genotype and allele frequencies of this gene variant was also not significantly different among gender and ethnic groups. On the basis of the results available so far, the role of the coding missense substitution Arg51Gln gene variant of Ghrelin in obesity, metabolic syndrome and their related traits remains inconclusive.
ACKNOWLEDGEMENTS
- This project was funded by the Universiti Tunku Abdul Rahman Research Fund (IPSR/RMC/UTARRF/C111/C35). We would like to extend our deepest gratitude to the Kampar District Health Office for granting us permission to carry out this study at the Kampar Health Clinic, the nurses who assisted with the blood sampling, and all the respondents who have volunteered to participate in this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML