-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Diabetes Research
p-ISSN: 2163-1638 e-ISSN: 2163-1646
2012; 1(3): 32-35
doi: 10.5923/j.diabetes.20120103.01
Quercetin Dampens Postprandial Hyperglycemia in Type 2 Diabetic Patients Challenged with Carbohydrates Load
Hussain S. A. 1, AhmedZ. A. 2, Mahwi T. O. 3, Aziz T. A. 4
1Department of Pharmacology and Toxicology, College of Pharmacy, University of Baghdad, Baghdad, Iraq
2Department of Pharmacology, School of Medicine, Faculty of Medical Sciences, University of Sulaimani, Kurdistan, Iraq
3Department of Internal Medicine, School of Medicine, Faculty of Medical Sciences, University of Sulaimani, Kurdistan, Iraq
4Department of Pharmacology, School of Pharmacy, Faculty of Medical Sciences, University of Sulaimani, Kurdistan, Iraq
Correspondence to: Hussain S. A. , Department of Pharmacology and Toxicology, College of Pharmacy, University of Baghdad, Baghdad, Iraq.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Postprandial hyperglycemia is a major risk factor for diabetic complications leading to disabilities and mortality in diabetics. Quercetin, a flavonoid, has been tried in traditional medicine for treating many disorders including diabetes. The present study was designed to evaluate the potential of quercetin to damp postprandial blood glucose level after maltose and glucose loading in patients with type 2 diabetes mellitus (DM). In a randomized, blinded crossover study, single oral dose (400mg) of quercetin or placebo formula was administered to the patients 30 min before loading with either maltose (2g/kg) or glucose (100g), blood samples are taken at different intervals for measurement of plasma glucose. The results clearly showed ameliorated postprandial hyperglycemia due to the use of quercetin, it significantly dampened the postprandial hyperglycemia only after maltose loading compared to placebo; no effect reported for quercetin after glucose loading. In conclusion, quercetin effectively suppresses postprandial hyperglycemia in patients with type 2 DM loaded with maltose, which may be attributed to α-glucosidase inhibition.
Keywords: Quercetin, Type 2 DM, PPGE, Glucose Absorption
Article Outline
1. Introduction
- Type 2 diabetes is characterized by two main features: peripheral insulin resistance and beta-cell dysfunction. Both hereditary and environmental factors, such as obesity and prolonged hyperglycemia, may trigger or exaggerate human type 2 diabetes. Hyperglycemia causes both beta-cell damage and peripheral insulin resistance via multiple mechanisms, collectively referred to as glucotoxicity[1]. The acute glucose fluctuations during the postprandial period exhibits a more specific triggering effect on oxidative stress than chronic sustained hyperglycemia which suggests that the therapy in type 2 diabetes should target not only hemoglobin A1c and mean glucose concentrations but also acute glucose swings[2]. In MODY-2 diabetes, functional defects in glucokinase genes restrict hepatic glucose uptake, bringing about prolongation of postprandial hyperglycemia[3], and eventually result in beta-cell overload. Hence, efforts to minimize postprandial hyperglycemia, as well as fasting blood glucose control, are of importance for the prevention and treatment of type 2 diabetes. Oxidative stress, which is the result of either the overproduction of freeradicals or some impairment in the cellular antioxidant systems, can lead to serious deterioration of health. For instance, oxidative stress is likely to be involved in both the development and the advancement of diabetes mellitus, as several studies have demonstrated the involvement of oxygen free radicals (reactive oxygen species) in the appearance of insulin resistance, a distinctive characteristic of type 2 diabetes[4,5]. In addition, oxidative stress might even play some direct role in the subsequent surfacing of other diabetes- related complications[6]. Quercetin (3,3΄,4΄,5-7-penta- hydroxyflavone), is a flavonol chemically related to kaemferol. As a member of the flavonoid family, quercetin is widely distributed in plants, and is probably the most abundant of the flavonoid molecules in the plant kingdom. Sources of quercetin include brassica green vegetables, berries, onions, parsley, apple, legumes, green tea, citrus fruits, red grape wines, and so on[7]. Quercetin prevents oxidative injury and cell death[8] by several mechanisms, including scavenging oxygen radicals[9], inhibiting xanthine oxidase[10], lipid peroxidation, and chelating metal ions[11]. Quercetin is an efficient antioxidant, as evidenced by both in vitro[12] and in vivo studies[13], and in parallel, it has also been shown to improve diabetes-related impairments in animals[14,15]. Thus, in the present study, we address the question whether quercetin has beneficial effects in controlling and/or improving postprandial hyperglycemia in type 2 DM patients challenged with mono- or disaccharide load.
2. Materials and Methods
2.1. Selection of Patients
- Twenty four glibenclamide-treated type 2 diabetic patients were enrolled in this study. The study protocol was approved by the Ethical Committee of the College of Medicine/University of Sulaimani and carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000, and all patients gave informed consent. No subjects were taking any medications that influence glycemic control other than glibenclamide, and all abstained from alcohol, tobacco, and strenuous physical activity for 24 hrs and caffeine-containing drinks overnight.
2.2. Study Protocol
- The patients were randomized into two groups of (12 in each), the effect of either placebo formula or single 400mg quercetin dihydrate oral dose on postprandial glucose excursions after loading with either 2g/kg maltose or 100g glucose were evaluated in a crossover design. After overnight fasting, either single 400mg quercetin dihydrate (Xian Keen, China), specially formulated for this purpose, or placebo was orally administered, followed (after 30 min) by loading of maltose or glucose. The order to receive capsules (placebo or quercetin) was randomized, and the kind of capsules taken was blinded to the examiner. Blood samples were obtained at 0, 30, 60, 90, 120, 150 and 180 min in tubes containing EDTA sodium (1 mg/ml) and immediately separated for plasma by centrifugation at 3000 rpm at 4°C for 10 min. Routine chemical methods were used to determine the plasma concentrations of glucose[16].
2.3. Statistical Analysis
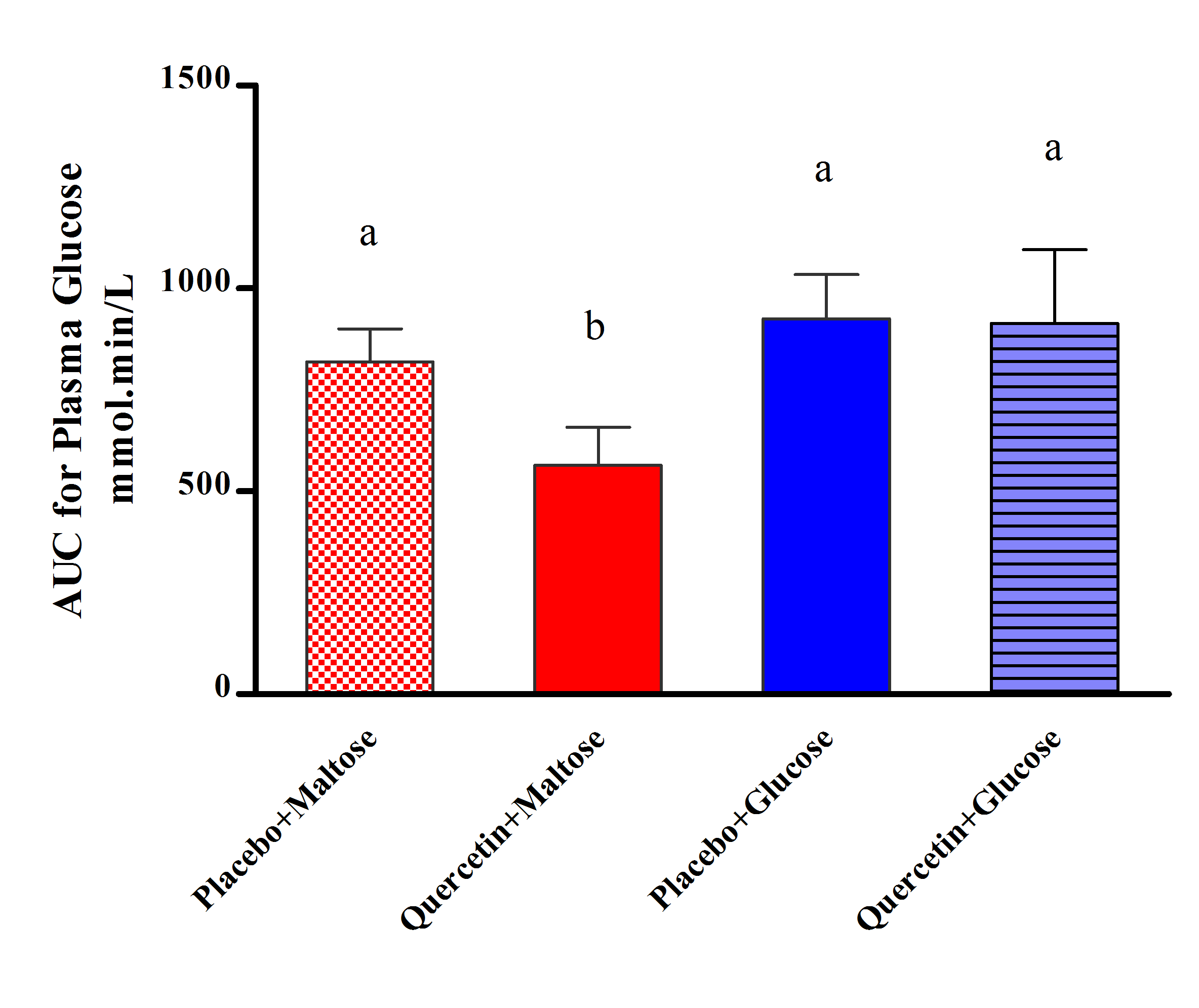
- Values are expressed as the mean ± SD. Area under the curve (AUC) that represents the relation between the change in plasma glucose with respect to baseline and time was estimated. Two-tailed unpaired Student’s t test or one-way factorial ANOVA, followed by Bonferroni’s post hoc comparisons, was used to compare means of AUC. P<0.05 was considered statistically significant. Analyses were processed using GraphPad Prism software for Windows (version 5.0, GraphPad Software, Inc., San Diego, CA).
3. Results
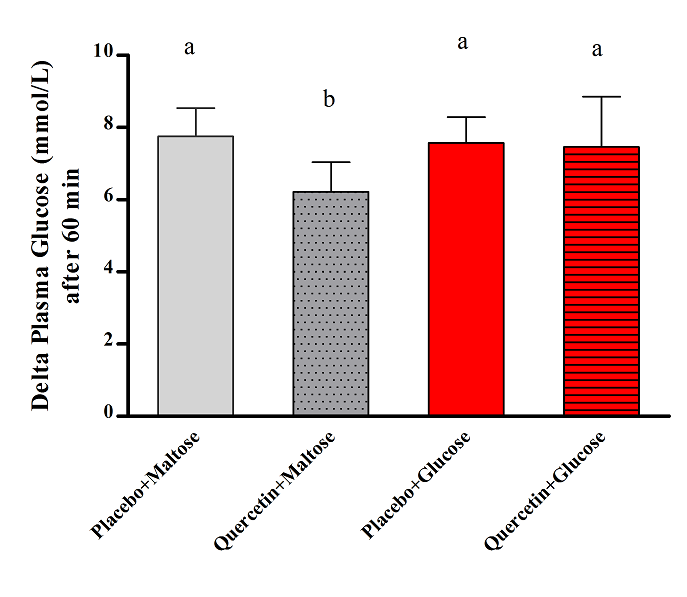
- Figure 1 showed that quercetin did not produce apparent changes on the rate of postprandial hyperglycemia after glucose load, where the changes in plasma glucose levels appeared comparable in both quercetin and placebo treated groups. Meanwhile, figure 2 clearly showed that administration of 400mg quercetin, as single oral dose before maltose challenge, decreased the magnitude of glucose spikes during 3 hrs follow up of glucose levels compared to placebo group.
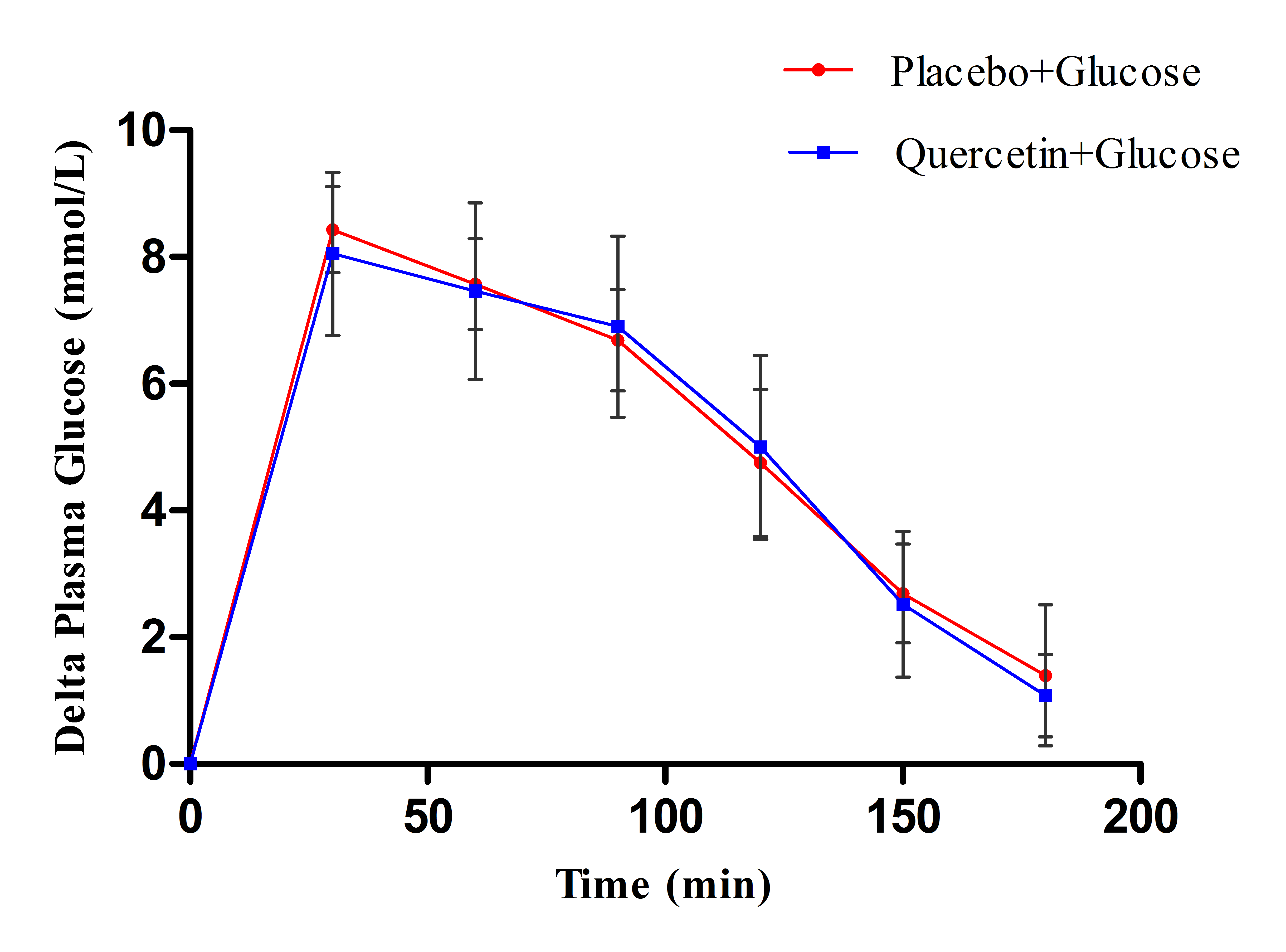 | Figure 1. Effect of single oral dose (400mg) of quercetin and placebo on the change in plasma glucose levels after 100 g glucose load in type 2 DM patients |
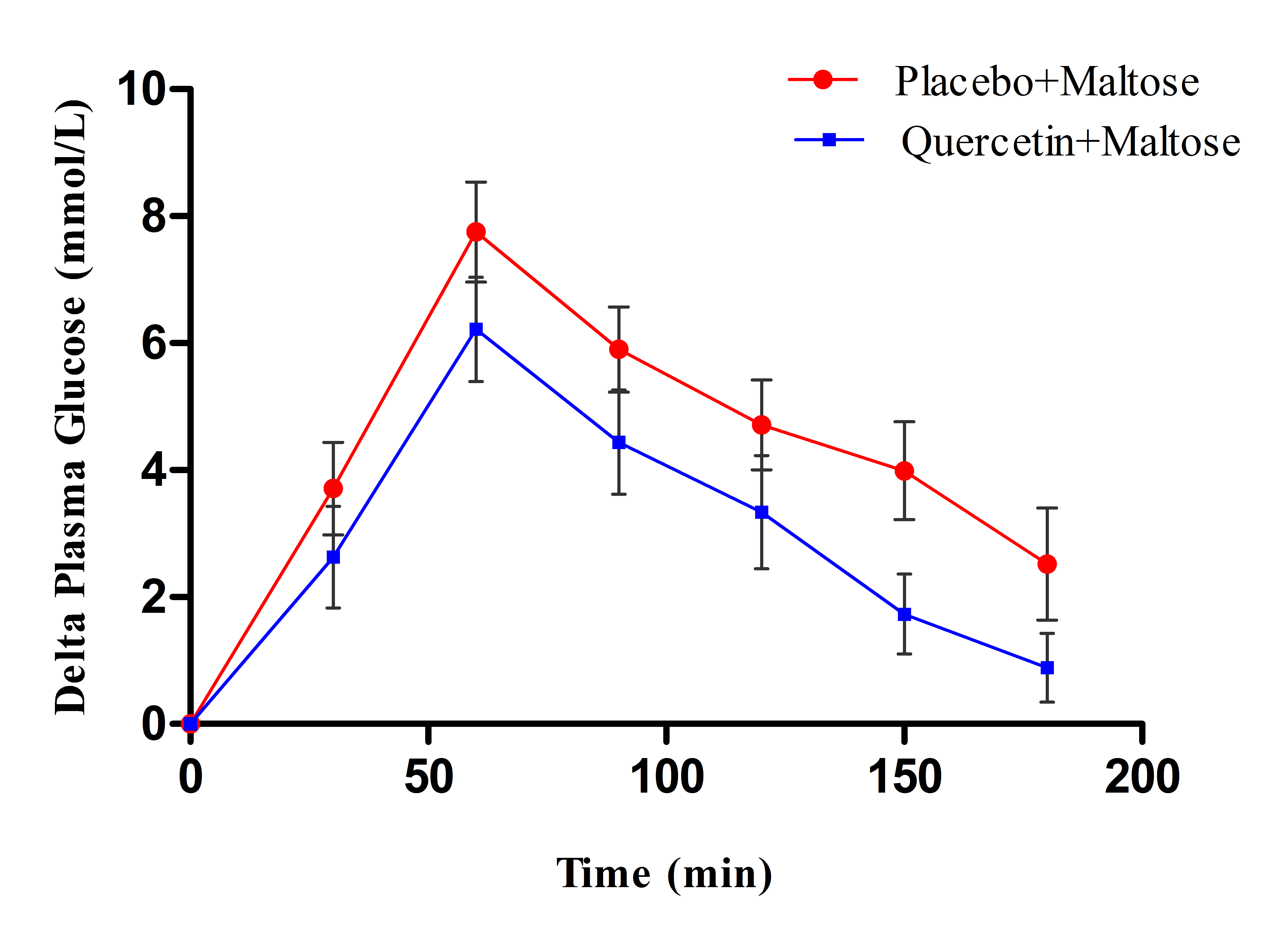 | Figure 2. Effect of single oral dose (400mg) of quercetin and placebo on the change in plasma glucose levels after 2g/kg maltose load in type 2 DM patients |
4. Discussion
- Controlling not only fasting, but also postprandial hyperglycemia, is important in achieving tight control of blood glucose levels, which is the major target of diabetic therapy[17]. It was reported that postprandial hyperglycemia might be more strongly correlated with cardiovascular morbidity and mortality than fasting hyperglycemia[18]. Postprandial hyperglycemia has been shown to increase the production of free radicals, which induce vasoconstriction and stimulate prothrombotic pathways leading to an increased risk of cardiovascular disease, the major cause of premature death among type 2 diabetic patients[19]. Although α-glucosidase inhibitors are common oral hypoglycemic agents, the chronic use of these agents can lead to gastrointestinal side effects such as flatulence, vomiting, and diarrhea[20]. Thus, many investigators have performed studies to discover α-glucosidase inhibitors from natural products with reduced side effects[21,22]. Among known materials with α-glucosidase inhibitor activity, guava leaf extract and Touchi extract have been approved as individual authorized health functional foods to improve postprandial hyperglycemia in Korea, and as Foods for Specified Health Use (FOSHU) in Japan[23]. However, few flavonoid drugs have reached clinical application so far, mostly due to the pleiotropic effects inherent to this class of substances and difficulties to anticipate side-effects. Quercetin is an exception since it has already been used therapeutically for a long time. Thus, there is considerable amount of data about its efficiency, pharmacokinetics, and safety[24]. In the present study, the data indicated that quercetin can decrease postprandial glucose level after disaccharides loading, which may be mainly attributed to inhibition of α-glucosidase as one of the expected mechanisms for the reduction of plasma glucose; this effect subsequently lead to suppression of postprandial hyperglycemia. Thus, quercetin can be considered as a potential candidate for the management of type 2 diabetes mellitus by this mechanism. Medicines that reduce postprandial hyperglycemia by suppressing the absorption of carbohydrates are shown to be effective for prevention and treatment of non-insulin-dependent diabetes mellitus[25]. Many naturally occurring flavonoids have hypoglycemic potential and have been described as glucosidase inhibitors; they are considered as a promising source for drug development and have shown impressing biological effects in vitro and in vivo[26]. While the antioxidant activity is regarded as the main mechanism underlying the biological effects of quercetin in many diseases, recent evidence suggests that quercetin could be beneficial in the treatment of type 2 diabetes owing to its anti-hyperglycemic properties[15]. Recent studies have shown that many flavonoids including quercetin and cyanidin-3-glucoside inhibit in vitro the intestinal α-glucosidase activity[27,28]. Considering the data obtained from our investigation, quercetin may play similar role in controlling postprandial hyperglycemia by inhibiting intestinal α-glucosidase in humans with type 2 DM. According to literature evaluation about flavonols and flavones, it can be assumed that quercetin may interact with protein by using hydroxyl groups in their molecular structure, resulting in the formation of hydrogen bonds with the polar groups (amide, guanidine, peptide, amino and carboxyl groups) in the active site of proteins by covalent and/or non-covalent interactions. To prove this hypothesis, X-ray crystallography and computer modeling to evaluate the binding activity of quercetin on intestinal α-glucosidase is needed for further investigation. Although further study is required for the kinetic of the enzyme inhibition, encouraging data were previously available concerning the inhibitory effects of many flavonoids on α-glucosidase enzyme; although these findings were incomplete, but they can describe the hypoglycemic effect of flavonoid by inhibition of intestinal glucosidase activity[26]; accordingly, the finding of the present study is compatible with this assumption. We have also evaluated the effect of quercetin on glucose loading in diabetic patients. Quercetin, when administered as single oral dose, do not suppress the postprandial hyperglycemia associated with glucose loading similar to that of maltose loading; this indicates that the major mechanism of action of postprandial glucose suppression is attributed only to the inhibition of α-glucosidase, but the involvement of other mechanisms can not be ruled out when multiple doses used for longer period of time. The reported results of the current study may provide preliminary evidence about quercetin action that could serve as a base for a pilot clinical study in this respect. In the present study, the observed reduction in maltose-induced postprandial hyperglycemia following treatment of type 2 diabetic patients with quercetin, confirm the potential role of this flavonoid to inhibit intestinal α-glucosidase activity; the failure of quercetin to damp glucose-induced glycemic spikes confirm the involvement of this mechanism in controlling postprandial hyperglycemia. In conclusion: orally administered quercetin as a single dose in patients with type 2 DM dampen postprandial hyperglycemia induced by maltose load, while has no effect when diabetic patients are loaded with glucose.
ACKNOWLEDGEMENTS
- The presented data were abstracted from PhD thesis submitted to the Department of Pharmacology, School of Medicine, University of Sulaimani. The authors thank University of Sulaimani for supporting the project
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML