-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Practice
p-ISSN: 2326-1463 e-ISSN: 2326-1471
2017; 6(2): 37-45
doi:10.5923/j.cp.20170602.04

Evaluation of Left Ventricular Deformation and Its Correlation with Markers of Inflammation in Recent Onset Rheumatoid Arthritis Patients
Rehab M. Hamdy1, Marwa M. Hassan2, Fatma A. Attia2, Nagwa A. Mohamed3, Abeer M. Shawky1
1Al-Azhar University, Department of Cardiology, Cairo, Egypt
2Al-Azhar University, Department of Internal Medicine, Cairo, Egypt
3National Research Centre, Clinical and Chemical Pathology Department, Cairo, Egypt
Correspondence to: Rehab M. Hamdy, Al-Azhar University, Department of Cardiology, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this study: was to evaluate left ventricular (LV) deformation and its correlation with inflammatory markers in recent onset rheumatoid arthritis patients. Patients and Methods: the study enrolled 50 rheumatoid arthritis (RA) patients without clinically identified cardiovascular disease (CVD) with disease duration ≤ 24 months whom were compared to 40 age and sex matched healthy controls. The disease activity was assessed according to disease activity score (DAS-28). Patients were reclassified into 2 groups; group A (moderate to severe RA) and group B (low disease activity or remission). Patients and controls underwent echocardiographic study including 2D-STE and laboratory assessment including ESR, CRP and angiopoietin-2 (ang-2). Results: LV global longitudinal strain (LV-GLS) was reduced in early onset RA patients compared to controls (17.7±2.6% vs. 20±2%, p <0.0001). LV-GLS was further impaired in group A compared to group B(16.8±2.1 vs. 18.7±2.8%, p<0.01). The serum level of ang-2 was higher in RA patients compared to controls (2717.8±1091.3 vs. 462.6±120.7 pg/ml, p<0.001) and ang-2 level was markedly elevated in group A compared to group B (3633.4±502.3 vs. 1642.9± 344 pg/ml, p<0.0001). LV-GLS correlated negatively with DAS-28 score (r=-0.388, p<0.05) and with inflammatory markers as ESR (r=- 0.317, p<0.05) and CRP (r=-0.344, p<0.05) and ang-2 (r=-0.347, p<0.05). Conclusions: Early onset RA patients without identified CVD have subclinical impairment of LV systolic function using 2D- STE that was further reduced in patients with higher levels of inflammatory markers signifying the impact of inflammation on the myocardium. Deformation imaging may represent an effective tool for detection of subclinical LV dysfunction and identification of high risk patients.
Keywords: Rheumatoid arthritis, 2D-STE, Inflammatory markers, ang-2
Cite this paper: Rehab M. Hamdy, Marwa M. Hassan, Fatma A. Attia, Nagwa A. Mohamed, Abeer M. Shawky, Evaluation of Left Ventricular Deformation and Its Correlation with Markers of Inflammation in Recent Onset Rheumatoid Arthritis Patients, Clinical Practice, Vol. 6 No. 2, 2017, pp. 37-45. doi: 10.5923/j.cp.20170602.04.
Article Outline
1. Introduction
- Rheumatoid arthritis (RA) is a systemic autoimmune disease affecting about 1% of the general population. It is characterized by chronic inflammation and enhanced atherosclerosis [1]. Patients with RA are at increased risk for developing cardiovascular disease (CVD), including a two-fold risk of heart failure (HF) [2] and the risk of cardiovascular (CV) mortality up to 50% compared with the general population [1].Increased incidence of CV events in RA is mainly a consequence of accelerated atherogenesis [3] that cannot fully be explained by traditional CV risk factors [4]. Accelerated atherogenesis accompanying RA is linked to endothelial activation and dysfunction [5] that has been observed already in patients with early RA [6]. Endothelial dysfunction is considered a consequence of a corollary of multiple interactions including elevated inflammatory activity which appears a major contributor to endothelial dysfunction in patients with RA [7]. Angiopoietin-2 (ang-2), a marker of endothelial cell activation might play an important role in the regulation of endothelial integrity and inflammation [8].RA patients who develop clinical HF often have impaired left ventricular (LV) diastolic function with preserved LV ejection fraction (EF), and may present with relatively fewer signs and symptoms in comparison to HF patients without RA [9]. Disturbances of diastolic function precede systolic HF and, although clinically silent, represent the earliest sign of cardiac involvement [10]. However, screening RA patients for CVD remains controversial, and the best method for evaluation of subclinical disease remains unclear [11].Myocardial strain imaging may be well suited for early detection of CVD in RA. Speckle tracking echocardiography (STE) is a peculiar technique of 2- dimensional (2D) echocardiography images analysis. It has been introduced to allow the study of myocardial deformation [12].Fine et al., [11] found global longitudinal systolic strain (GLS) to be reduced in patients with long-standing RA compared to controls. It would be of great interest to detect even earlier evidence of subtle cardiovascular injury in recently diagnosed onset RA patients.The purpose of this study was to evaluate LV deformation using different echo-Doppler modalities and its correlation with markers of inflammation in recent onset rheumatoid arthritis patients without clinically identified CVD.
2. Patients and Methods
2.1. Study Population
- Cross-sectional study enrolled 50 RA patients with disease duration ≤ 24 months and without clinically identified CVD. The study recruited patients from Rheumatology outpatient clinic Al-Zahraa University hospital (Cairo, Egypt) in the period between August 2016 and February 2017. RA patients were compared to 40 age and sex matched healthy volunteers as a control group. All patients with identified heart disease (ischemic heart disease, congestive heart failure, significant valvular heart disease, pericardial disease, congenital heart disease or significant arrhythmia) were excluded from the study. All patients with advanced comorbidities that might alter left ventricular functions (e.g. advanced liver failure, chronic kidney disease, severe obstructive pulmonary disease, hypo- or hyperthyroidism, clinical history and signs or symptoms of cerebrovascular events) were also excluded from this study. We exclude patients with technically poor acoustic window precluding satisfactory 2D echocardiographic imaging of the LV. Patients were diagnosed according to The 2010 European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) criteria [13]. The study was approved by our ethics committee of the faculty of medicine, and an informed consent was taken from every subject.All patients and controls were subjected to full history taking, full clinical examination especially musculoskeletal examination. Mean duration of the disease was 13.7±7.5 months (2-24 months).Disease activity was assessed using 28-joint disease activity score (DAS-28). The DAS 28 was calculated as following:DAS28=0.56x√TEN28+0.28x√SW28+0.70xIn (ESR) +0.014xSATEN=tender joint, SW=swollen joint, ESR= erythrocyte sedimentation rate and SA=Patients subdivided into 4 groups; DAS 28 > 5.1 = high disease activity, DAS 28 > 3.2 < 5.1 = moderate activity, DAS 28 > 2.6 < 3.2 = low disease activity, DAS 28 < 2.6 = remission [14, 15].All patients received anti-rheumatic therapy (conventional synthetic or biological disease modifying anti-rheumatic drugs or steroids or non-steroidal anti-inflammatory drugs).The study population was classified into:Group A (moderate to severe RA): included RA patients with DAS 28>3.2 Group B (low activity RA or remission of the disease): included RA patients with DAS28<3.2.
2.2. Laboratory Assessment
- Blood samples were collected after an overnight fast in all patients and controls and were analyzed for total cholesterol, triglycerides, fasting blood glucose, liver functions, blood urea nitrogen, serum creatinine, ESR and CRP.Immunological profile was performed for detection of rheumatoid factor (RF), anti-cyclic citrullinated peptide antibody (ACPA) and serum levels of ang-2.
2.3. Echocardiography
- All echocardiographic examinations were performed by a single operator. Transthoracic echocardiographic (TTE) images were recorded using the VIVID S5 system (General Electric Vivid S5; GE Vingmend Ultrasound AS, Horten, Norway) with 3S-RS phased array sector probe (1.5-3.6 MHz) with tissue Doppler imaging (TDI) capability. Comprehensive trans-thoracic M-mode, 2Dimensional (2D), and Doppler were done in standard views to measure left ventricular (LV) dimensions, LV volumes. The LV ejection fraction (EF) was calculated by a modified biplane Simpson's method.Doppler indices of LV diastolic function were measured using standard techniques [16]. The transmitral early (E wave) and late (A wave) diastolic velocities and E/A ratio were recorded.A) Tissue Doppler Imaging (TDI)The tissue Doppler velocity profiles were derived from the 3 apical views with Doppler sample volume placed at septal, lateral, anterior, inferior, posterior and antroseptal mitral valve annuli. The following parameters were measured for each site: average peak systolic mitral annular velocity (Sm), early diastolic annular velocity (Em) and late diastolic annular velocity (Am) then E/Em ratio which is the average peak early transmitral diastolic velocity measured by Doppler (E)/ the average peak early diastolic velocities at 6 mitral annular sites measured by TDI as indicator of LV diastolic function. B) Speckle tracking analysisSpeckle tracking analysis was performed off-line applying a commercially available LV strain software package to the left ventricle (EchoPAC version 110.1.2). Using dedicated software package Automatic Function Imaging (AFI), 2D images were obtained from the 3 apical views, and storing three cardiac cycles in cine-loop format for off-line analysis to assess LV longitudinal strain by tracing the endocardial border on an end-diastolic frame and the software automatically tracked the border on the subsequent frames. The LV was divided into 17 segments and global LV longitudinal strain (LV-GLS) were calculated. A cut-off point for LV-GLS was set to 20.0% [17]. Low LV-GLS value than 20% means impaired LV systolic function.
2.4. Statistical Analysis
- Results were analyzed using the SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as percentages, means and standard deviations (SD). The parameters with normal distribution were expressed as the mean 1 SD. Univariate analysis for group comparisons were performed using the Student’s t-test. The associations between variables were assessed by Chi-Square test, Pearson and Spearman’s r correlation analysis. P < 0.05 was accepted as statistically significant.
3. Results
3.1. Baseline Criteria and Inflammatory Markers among the Study Population
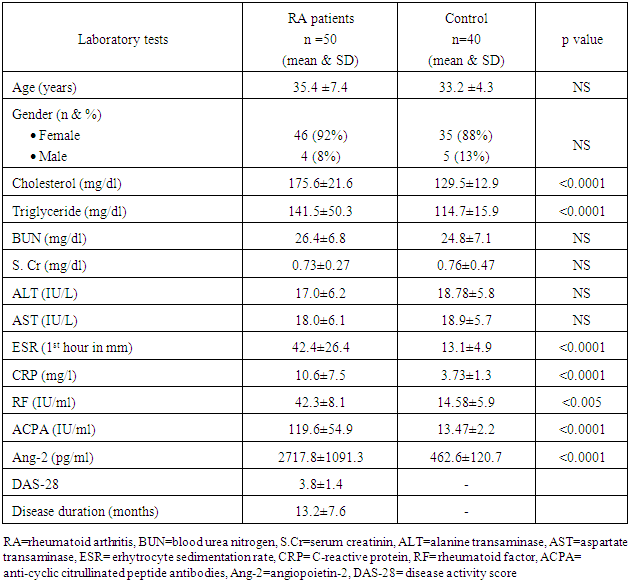
- The Baseline characteristics of the studied population are summarized in table (1). The study population consisted of 50 RA patients; 4 males (8%) and 46 female (92%) with their mean age 35.4 ±7.4 years compared to 40 healthy subject; 5 male (13%) and 35 female (88%) with their mean age 33.2±4.3 years. Both groups were comparable regarding age, sex, lipid profile, renal and liver functions (table 1). RA patients had significantly higher serum levels of cholesterol and triglyceride compared to control group. Inflammatory markers (ESR, CRP, RF, ACPA and ang-2) levels were significantly elevated in RA patients compared to healthy control (table 1). However, no significant differences between both groups regarding age, sex, renal (serum creatinine and blood urea nitrogen) or liver functions (ALT and AST).
|
3.2. LV Dimensions and Functions among the Study Population
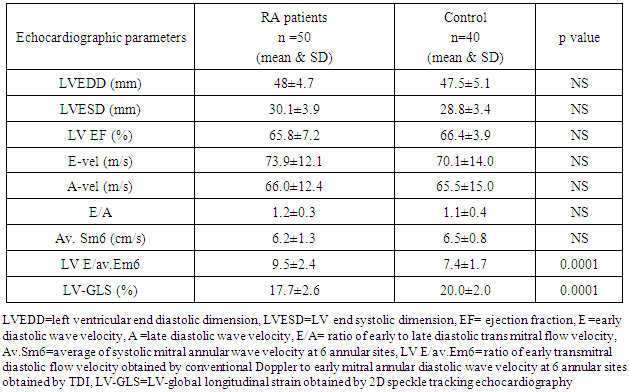
- We found that RA patients had significantly lower LV-GLS (17.7±2.6% vs. 20.0±2.0%, p<0.0001) and higher LV E/av.Em6 (9.5±2.8 vs.7.4±1.7, p<0.0001) compared to healthy controls while no significant differences were found in the remaining echo-Doppler parameters between the RA and healthy controls as shown in (table 2) (figure 1).
|
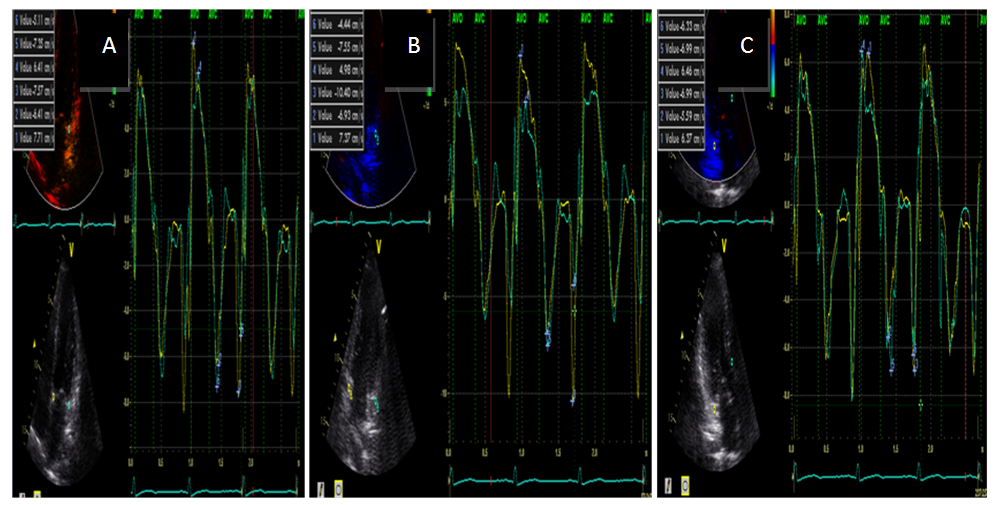 | Figure (1). TDI derived systolic, early and late diastolic annular velocity at (A) septal and lateral (B) inferior ad anterior (C) posterior and antroseptal sites in RA patient |
3.3. Comparison between Group A (Moderate to Severe RA) and Group B (Low Disease Activity or Remission)
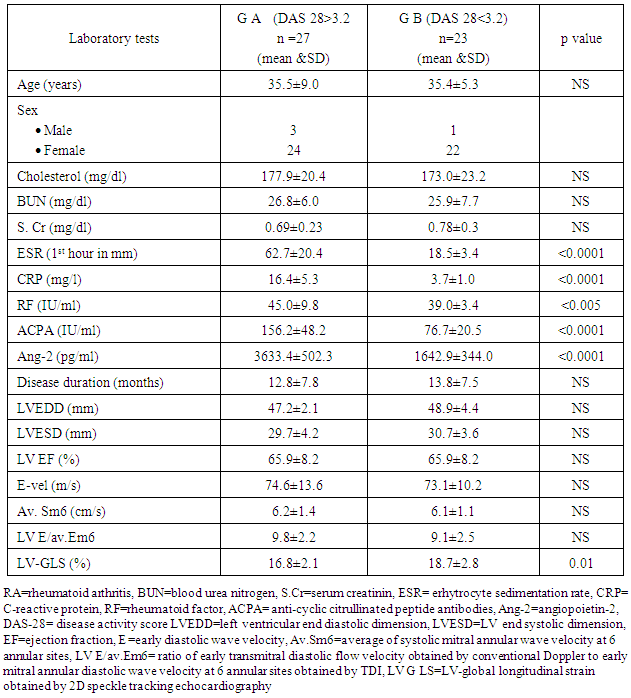
- Patients were classified according to the severity of the disease into group A (moderate to severe RA) that included 27 patients (DAS 28 > 3.2) with mean age 35.5 ± 9.0 years, group B (low disease activity or remission) that included 23 patients (DAS 28 <3.2) with mean age 35.4±5.3 years .We found higher levels of inflammatory markers (ESR, CRP, RF, ACPA and ang-2) in group A compared to group B (62.7±20.4 vs. 18.5±3.4, 16.4±5.3 vs. 3.7±1.0, 45.0±9.8 vs. 39.0±3.4, 156.2±48.2 vs. 76.7±20.5 and 3633.4±502.3 vs. 1642.9±344.0 respectively) (table 3). Otherwise, no significance differences were found between both groups regarding age, sex disease duration and the remaining laboratory tests (table 3).By comparing echo-Doppler parameters between both groups, we found that only LV-GLS value was significantly reduced in group A compared to group B (both were below the cutoff point (20%) (table 3) (figure 2).
|
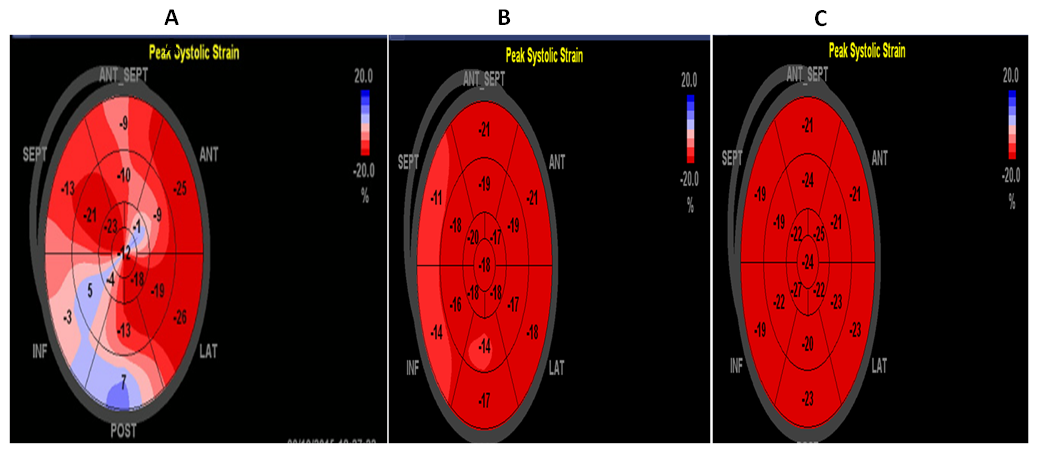 | Figure (2). Ball's eye of 17-segments of LV-GLS in (A) RA patient with DAS=4.5, LV-GLS=13.1%, (B) RA patient with DAS=2.9, LV-GLS= 17.3% and (C) control volunteer, LV-GLS=22% |
3.4. Correlation between LV-GLS Assessed by 2D-STE with Inflammatory Markers and DAS-28 Score
- We found significant negative correlation between LV-GLS and the levels of the following inflammatory markers (ESR, CER, and ang2) among RA patients. Moreover, LV-GLS was negatively correlated with DAS-28 score (figure 3). We could not find significant correlation between LV-GLS with RF or ACPA.
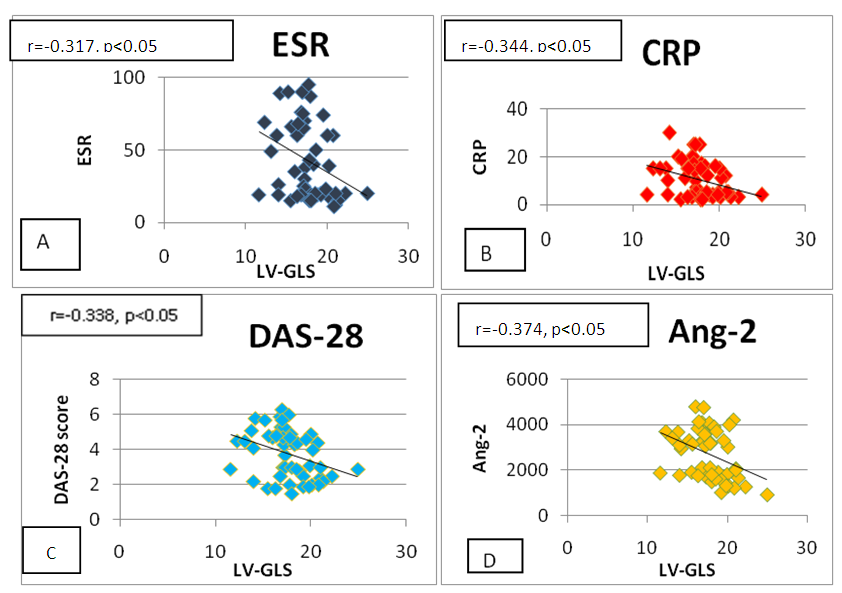 | Figure (3). Correlation between LV-GLS with (A) ESR (B) CRP (C) DAS-28 score and (D) Ang-2 |
3.5. Correlation between DAS-28 Score with Inflammatory Markers among RA Patients
- DAS-28 score correlated positively with the following inflammatory markers: ESR (r=0.919, p<0.001), CRP (r=0.940, p<0.001), RF (r=0.313, p<0.05), ACPA (r=0.640, p<0.001) and ang-2 (r=0.828, p<0.001).
4. Discussion
- Despite remarkable improvements in RA treatment, there is evidence indicating that the mortality gap between patients with this disease and the general population is not closing. This increase in mortality in RA is predominantly due to accelerated coronary artery and cerebrovascular atherosclerosis, as well as to other CV complications including HF [2]. There is considerable evidence suggesting that chronic inflammation contributes to impairment of endothelial and microcirculatory function, leading to the early onset of atherosclerosis in RA patients [18, 19]. This perfusion impairment may lead to myocardial systolic and diastolic dysfunction. Moreover, the excess of CV mortality and morbidity could be explained by disease duration, disease activity, immunosuppressive therapy in addition to traditional CV risk factors [20]. Inflammatory and serological markers such as ESR, CRP, RF, and ACPA have been positively associated with increased atherogenicity and CV events in several studies assessing RA patients [21] as well as in the general population [22]. Ang -2 causes vascular destabilization, thereby rendering the endothelium responsive to stimulation by inflammatory and angiogenic cytokines [23].Prolonged inflammation may lead to oxidative stress, myocyte dysfunction [24] and cytokine-induced increase in fibroblast activity causing myocardial collagen deposition and interstitial fibrosis [1]. The subsequent accelerated atherosclerosis and functional abnormalities of the endothelium suggest a subclinical CV involvement beginning rapidly soon after the onset of the disease at younger age than in general population and progressing with the disease duration [25].Several echocardiographic studies have demonstrated left ventricular systolic and diastolic dysfunction in RA patients [26]. Most of these studies were conducted using conventional techniques such as Doppler echocardiography, color Doppler M-mode, and TDI. Although TDI is considered a reliable tool for the assessment of myocardial deformation, the angle-dependent nature of the method constitutes a limitation. While the myocardium deforms simultaneously in three dimensions, the TDI can only measure the deformation along the ultrasound beam from the myocardial tissue velocities. 2D-STE is a peculiar imaging modality that is angle independent and tracks the myocardial strain along the direction of the myocardial wall in 2D images [27].In the current study, we found no significant differences in LV dimensions and LV EF assessed by conventional echocardiography between the RA and healthy controls. Consistent to our results, Sitia et al, [28]; who evaluated the LV function in 22 RA patients with short disease duration by conventional echocardiography. They found that RA patients had normal LV diameters, volumes, and systolic function with no statistically significant differences compared to healthy controls. Fine et al, [11] studied 87 patients with RA and concluded no significant difference was found between RA patients and the control group in left ventricular dimensions or LV ejection fraction.Discordant to our results, Bharti et al, [29] demonstrated an impairment of LV systolic function, reflected by a reduced EF in adolescents with RA. In the present study, we did not detect any significant difference regarding E wave, A wave or E/A ratio between RA patients and control group. This could be explained on the basis of diastolic function is mainly dependent on myocardial relaxation and LV load [30]. Meune et al, [30] found similar findings of transmitral E and A velocities, and isovolumic relaxation time (IVRT) in the patients and controls, indicate that LV relaxation was not affected in the patients with RA. We found higher LV E/av.Em6 ratio (a surrogate marker for LV diastolic function) in RA patients compared to control group denoting that the patients with RA had higher LV-filling pressures than the controls but no significant difference of LV systolic function measured by TDI using the average systolic mitral velocities at 6 annular sites (Av. Sm6) between RA patients and control group.Concordant to our results, Aulie et al, [31] stated that diastolic function in RA patients was altered compared to controls which was characterized by higher marker of LV filling pressure (lateral E/Em). Meune et al, [30] concluded that TDI identifies impaired diastolic function in patients with RA that may not be detected by conventional measurements. Compared to our results, Cioffi et al, [32] found a significant reduction in LV systolic function of myocardial longitudinal fibers measured as Sm by TDI in RA patients compared to controls (9.3±1.7 cm/s vs. 10.5±2.1cm/s respectively, p < 0.001) and a higher E/Em ratio in RA patients compared to control (7.3±2.4 vs. 6.2±1.8, p<0.01).Sitia et al, [28] showed that RA patients had a significant reduction in (Sm) and (Em) with higher E/Em ratio compared to healthy controls by TDI parameters. In our study population, RA patients showed significant impairment of LV-GLS compared to healthy volunteers (17.7±2.6% vs.20.0±2.0%, p <0.0001 respectively). LV-GLS was further reduced among RA with moderate to severe disease activity compared to those with low activity or during remission (16.8±2.1% vs. 18.7±2.8% respectively). Parallel to our findings, Fine et al, [11] included 59 patients with RA compared with 59 age-, gender- and race-matched subjects with normal echocardiography and no CVD or risk factors. Global LV strain (15.7±3.2% vs 18.1±2.4%, p<0.001) significantly impaired in patients with RA compared with normal persons. They concluded that LV strain abnormalities correlated with RA disease severity.In agreements with our results, Sitia et al, [28] evaluated the LV function in 22 RA patients with short disease duration by echocardiography. They found a significant reduction in LV longitudinal and radial strain mainly of basal and mid septal, basal and mid lateral and apical segments in RA patients without coronary artery disease compared to health control.Ikonomidis et al, [33] studied 46 RA patients without CVD and compared them with 23 healthy controls. Significant differences in LV systolic longitudinal, circumferential and radial strain between RA patients and controls were observed.Midtbo et al, [34] studied 78 patients with RA without known cardiac disease presented with different grades of disease activity, 41 patients in remission and 46 controls. The study revealed that GLS and stress-corrected midwall shortening (scMWS) were reduced in patients with RA with active disease compared to patients with RA in remission. Patients with RA in remission had similar scMWS and GLS as the controls. Active RA is associated with lower LV systolic myocardial function (assessed by scMWS and LV-GLS) despite normal LV EF and independent of traditional cardiovascular risk factors.In the current study, there was a significant increase in serum levels of ESR, CRP, RF, ang-2 and ACPA among RA patients in comparison to controls. Moreover, higher levels of these inflammatory markers were found in patients with moderate to severe disease activity (DAS> 3.2) compared to those with low activity or in remission (DAS<3.2). DAS-28 score was positively correlated the following inflammatory markers; ESR, CRP, RF, ang-2 and ACPA. Also, we found significant negative correlation between LV-GLS with disease activity and inflammatory markers (including ESR, CRP and ang-2) in RA patients. We suggest that patients with severe activity of RA had higher levels of inflammatory markers and markedly reduced of LV systolic function defined by LV-GLS relative to those with mild form of RA or in remission.Omar et al, [35] evaluated 44 RA patients without clinically overt CVD and 44 healthy controls. DAS-28 was calculated and serum Ang-2 level was measured. The result revealed that mean levels of Ang-2 was higher in RA patients as compared to controls and was significantly elevated in RA patients with LV diastolic dysfunction than those without dysfunction. Shrivastava et al, [36] studied 110 RA patients and 55 controls matched by age and sex. Serum levels of high sensitive (hs)-CRP was estimated and correlated with the DAS-28. They found that RA patients had significantly higher levels of serum hs-CRP as compared to healthy controls and that hs-CRP was positively correlated with DAS-28. These results demonstrated that RA patients have high levels of inflammatory markers, and these levels are correlated with the DAS-28. These findings suggest a possible role of these markers in the pathogenesis of RA.
5. Conclusions
- Early onset RA patients without clinically identified CVD have subclinical impairment of LV systolic function defined by 2D-STE and impaired LV diastolic function defined by TDI. Further impairment of LV-GLS in patients with higher levels of inflammatory markers pointed out the direct impact of inflammation on the myocardium. Deformation imaging may represent an effective tool for detection of subclinical CVD and identification of high risk RA patients.
Limitations
- This is a cross sectional observational study included small sample size. The current findings require confirmation with larger sample size studies before myocardial deformation imaging recommended in RA patients. Also, we need to include RA patients with longer disease duration and those with CVD for further identification of adverse effects of RA on myocardial strain. We evaluated longitudinal strain of the LV but further research assessing all LV myocardial deformation are needed to determine the impact of RA disease progression on ventricular performance. Also, we suggest that further studies are needed to detect the effects of anti-rheumatic drugs on myocardial strain.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML