-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Practice
p-ISSN: 2326-1463 e-ISSN: 2326-1471
2017; 6(1): 9-13
doi:10.5923/j.cp.20170601.02

Asymptomatic Chronic Kidney Disease and Correlates in Untreated Hypertensive Patients Attending a Referral Hospital in Southern Nigeria
Maclean Romokere Akpa1, Ndubuisi Norbert Unamba2
1Department of Medicine, Faculty Clinical Sciences University of Port Harcourt, Port Harcourt, Nigeria
2Department of Medicine, University of Port Harcourt Teaching Hospital, Port Harcourt, Nigeria
Correspondence to: Maclean Romokere Akpa, Department of Medicine, Faculty Clinical Sciences University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

OBJECTIVE: The aim was to determine the prevalence of asymptomatic chronic kidney disease in untreated hypertensive patients referred to the cardiology clinic of UPTH based on the calculated eGFR using the Cockcroft-Gault equation. METHOD: A retrospective study of adult Nigerians presenting newly with essential hypertension only was conducted to determine the epidemiology of chronic kidney disease among them. RESULTS: A total of one hundred and sixty four subjects (164) treatment naïve hypertensive patients, 76 (46.3%) males and 88 (53.7%) females met the inclusion criteria. Mean age 52.88 ±15.02years. Mean BMI was 29.24±5.58Kg/m2, mean systolic blood pressure was 148 ± 21.9mmHg, and mean diastolic blood pressure was 91.36 ± 14.37mmHg. Mean serum creatinine was 117.01±114.27 mmol/L, and mean eGFR was 86.63 ±38.319 mls/min/1.73m2. 51.0% of subjects had stage I hypertension while 49.0% had stage II hypertension. Using the calculated eGFR, 55.49% of the patients had chronic kidney disease, 29.27% in stage II, 25% in stage III, and 1.22% in stage IV, while only 23 patients (14.02%) had serum creatinine level above the normal level. The eGFR correlated inversely but significantly with BMI and positively with serum uric acid. CONCLUSION: There is a high prevalence of undiagnosed chronic kidney disease in treatment naive hypertensive and normal serum urea and creatinine does not exclude the presence of CKD.
Keywords: Hypertension, Chronic kidney disease, Estimated glomerular filtration rate, Epidemiology
Cite this paper: Maclean Romokere Akpa, Ndubuisi Norbert Unamba, Asymptomatic Chronic Kidney Disease and Correlates in Untreated Hypertensive Patients Attending a Referral Hospital in Southern Nigeria, Clinical Practice, Vol. 6 No. 1, 2017, pp. 9-13. doi: 10.5923/j.cp.20170601.02.
Article Outline
1. Introduction
- Hypertension is the major non communicable disease among Nigerians [1] but awareness, treatment and control levels are generally very low as it is in other Sub Saharan African countries [2-4]. Complications are common [5] and may be present at first presentation. Chronic kidney disease (CKD) is common in Nigerians with an estimated adult prevalence ranging from 19% to 30% [6, 7] and several studies have documented hypertension as a major risk factor for chronic kidney disease [8, 9]. Prevalence of CKD is rising because of the rising prevalence of the major risk factors such as obesity, diabetes and hypertension and patients present late. Early chronic kidney disease is usually asymptomatic with long latent periods and only become symptomatic when at least 50% of functioning renal mass is lost [10]. CKD is believed to be commoner in Sub-Saharan Africa compared to the developed world and the epidemiology differs. CKD is found among the elderly population in the developed countries and diabetes mellitus is the commonest cause whereas in developing countries such as Nigeria it afflicts a younger age group (20-50 years) in their most productive age and most are due to hypertension [11]. Early detection of chronic kidney disease and associated risk factor and appropriate treatment has been advocated as the strategy needed to stem the rising prevalence of end stage renal disease [ESRD] in Nigeria and globally [12]. Early detection of CKD in hypertensive subjects would therefore allow for initiation of appropriate therapy to ensure blood pressure control to target using recommended drugs in other to retard progression of kidney disease. The epidemiology of CKD in newly diagnosed hypertensive patients is unknown but population based screening in Kano among Nigerians for CKD risks showed that hypertension constituted the highest risk factor at 29.8% [9]. When CKD becomes advanced, identification of etiology is difficult as renal biopsy may not be useful. The study was designed to determine the prevalence and degree of asymptomatic chronic kidney disease in newly diagnosed, untreated hypertensive patients seen in the cardiac clinic of the University of Port Harcourt Teaching Hospital, Southern Nigeria.
2. Methodology
- A cross sectional study carried out in the Cardiology Clinic of the Medical Out Patient Department of the UPTH over a two years period, January 2014 to December 2015. All the case notes of patients referred to the clinic because of elevated blood pressure were reviewed for biometric data, age se, and body mass indices. Blood pressure was measured with mercury in glass sphygmomanometer using standard protocol [13] and hypertension was defined and classified based on JNC 7 [14]. All the patients who were treatment naïve at their first visit and whose record showed results of full electrolytes, urea, creatinine, uric acid evaluation, fasting blood glucose and fasting lipids profile as well as urinalysis for proteinuria were selected for the analysis. One hundred and sixty four hypertensive patients ere recruited. This is the routine for all our patients in accordance with recommended guidelines [15]. Chemistry was carried out in the hospital laboratory using an autoanalyzer. Fasting serum total cholesterol, low density lipoprotein cholesterol and triglyceride levels were measured using the enzymatic method with a reagent from Atlas Medical Laboratories from the hospital’s central laboratory. LDL cholesterol values were calculated using the Friedewald equation when the triglyceride level was less than 4.0mmol/l: LDL = TC – (HDL + TG /2.2). [16] Urinalysis for sugar and protein was done with Medi-Test Combi 9Rdipstick [Macherey-Nagel, Germany]. Glomerular filtration rate [GFR] for all patients were calculated using the Cockcroft – Gault Equation [17] having been validated in Nigerians. All the patients who were previously diabetic or who had glucose abnormalities or glycosuria in their urinalysis results were excluded from the analysis.Chronic kidney disease was defined as eGFR less than 60mls/min/1.73m2, and based on the estimated GFR all patients were staged for chronic kidney disease. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines was used to stage participants for GFR categories of CKD [18].
2.1. Statistical Analysis
- The results were analyzed using the Statistical Package for Social Sciences (SSPS Inc, Chicago, IL) version 16 statistical software. For continuous variables, mean values and standard deviations were calculated and the means were compared using student t-test. Categorical variables were compared using the Chi-squares test. A P value<0.05 was used to characterize statistically significant results.
3. Results
3.1. Demographic Data
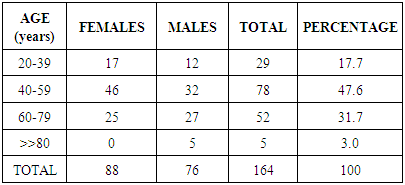
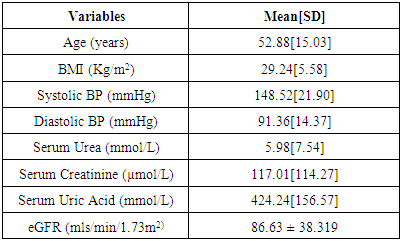
- A total of one hundred and sixty four subjects (164), treatment naïve hypertensive patients, 76(46.3%) males and 88 (53.7%) females [M: F of 1:1.2] met the inclusion criteria. Age range was 27 to 96years, mean age 52.88 ±15.02years. Mean body mass index (BMI) was 29.24±5.58Kg/m2, mean systolic blood pressure was 148 ± 21.9mmHg and mean diastolic blood pressure was 91.36 ± 14.37mmHg. Eighty four (51.22%) of the subjects had stage I hypertension while 80[48.78%] had stage II hypertension. Twenty- nine of the subjects (17.7%) had normal BMI, (BMI <25.0), 55(33.5%) were overweight, BMI 25 to 29.9Kg/m2, 57 [34.8%] were obese class I, BMI 30 - 34.9Kg/m2 15 (9.1%) were obese class II BMI 35.0 – 39.9Kg/m2 and 8(4.9%) were morbidly obese BMI >>40.0Kg/m2. The clinical and biochemical parameters are summarized in Table 2. The mean serum urea was 5.98±7.54mmol/L, mean serum creatinine was 117.01±114.27 mmol/L, and mean serum uric acid was 424.24±156.57µmol/L mean eGFR was 86.63 ±38.319 mls/min/1.73m2. 80% of all the subjects had increased serum uric acid level and among subjects with CKD 91.0% had elevated serum uric acid while only 21% of those without CKD have elevated uric acid.
|
|
3.2. Pattern of Kidney Disease
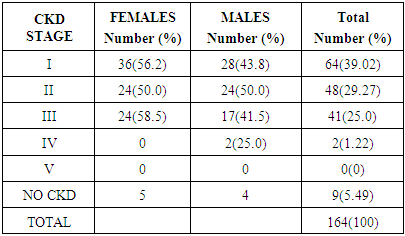
- CKD was present in a total of 23 subjects [14.02%] using serum creatinine level above the normal range (60-120µmol/L). Evaluating for CKD using the Cockcroft-Gault formula for estimated GFR (eGFR), revealed that 91 subjects (55.49%) had chronic kidney disease. The MDRD formula reported a CKD prevalence of 19.8% among the hypertensive subjects. Based on the estimated GFR, 64 patients [39.02%] were in stage I CKD, 48 patients (29.27%) were in stage II CKD, while 41 patients (25.0%) were in stage III CKD, 2 patient (1.22%) was in stage IV CKD and none was in stage V CKD (See Table 3).
|
 | Figure 1. Relationship between age and estimated GFR |
|
4. Discussion
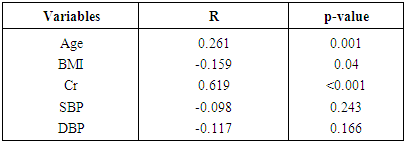
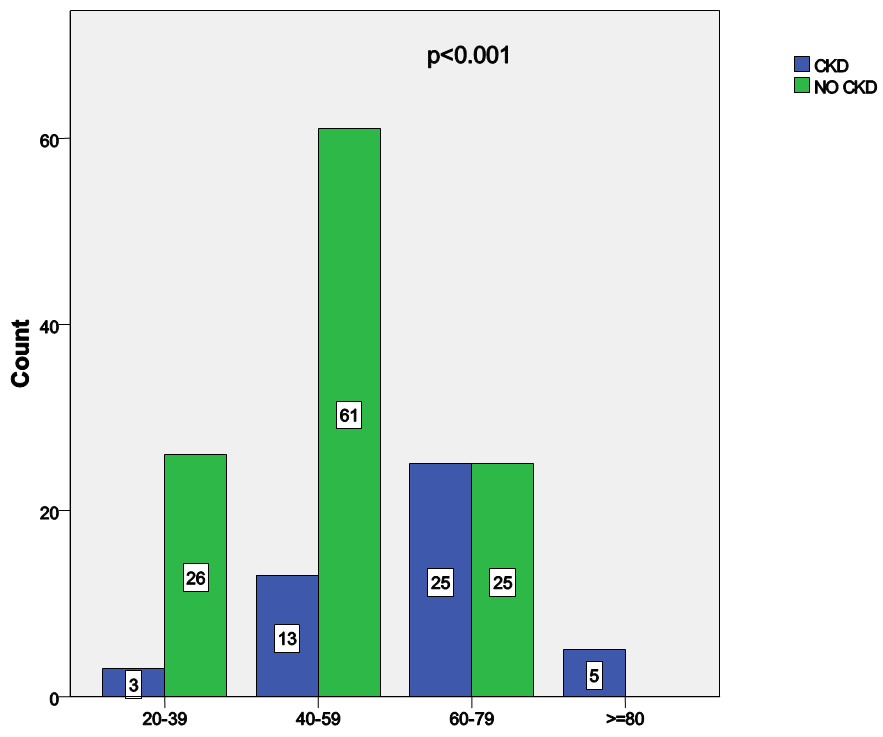
- This study showed that among newly diagnosed and treatment naïve hypertensive Nigerians seen in UPTH Port Harcourt during the study period, about 30% or one in every three have evidence of chronic renal disease using the calculated eGFR whereas based on serum creatinine only 23 patients (14.0%) had evidence of renal disease. This figure is lower than the 46.46% obtained from Ghana and Cameroun among patients who were hypertensive and receiving treatment and amongst whom there were diabetics [19, 20]. Hypertension is the leading risk factor for chronic kidney disease in Nigeria and treatment of hypertension to goal should reduce the burden of CKD by reducing the progression to ESRD. When the eGFR calculated from the Cockcroft – Gault equation was correlated with serum creatinine, 39.1% of those with CKD had elevated serum creatinine while 1.8% of those without CKD had increased serum creatinine above the reference level and the difference was statistically significant thus showing that eGFR is a sensitive screening tool for CKD in hypertensive subjects. The calculated eGFR allows for early identification of patients with renal dysfunction before abnormalities in serum creatinine is detected because reduced glomerular filtration rate [GFR] has been found to be a potent predictor of future cardiovascular events and death [21, 22] and may explain why the European Society of Hypertension recommends the calculation of estimated GFR in all hypertensive patients so that appropriate therapeutic antihypertensive measures can be undertaken. This is however more relevant in resource poor setting of Sub-Saharan Africa as Africans have been shown to have a higher risk of hypertensive renal disease, have renal involvement at a younger age also have a faster decline in renal function at similar blood pressure levels when compared to Caucasians [23]. When kidney disease develops in hypertensive patients, drug therapy and treatment goals require modification to retard progression of renal disease which is rising in parallel to the rise in hypertension prevalence. The eGFR did not show significant correlation with body mass index [BMI] using the Cockcroft – Gault calculation but using the MDRD calculation there was significant and inverse correlation between CKD and obesity (See Table 4). This agrees with the fact that MDRD identifies severe degrees of CKD and obesity has been shown in several studies to be an important risk factor for CKD as well as worsening CKD [24, 25]. In one of the largest surveys in a multiracial population, Hsu et al. [26] documented that body mass index (BMI) is strongly related to the risk of CKD. In their study, subjects with a BMI >40 kg/m2, i.e. the group with severe obesity, showed a risk for CKD seven times higher than that of the standard risk group with a normal BMI. This is due to the fact that obesity has been known to independently impact renal hemodynamics negatively by causing proteinuria and deteriorating renal function [27]. It has however been reported that renal haemodynamic alterations triggered by excess adiposity appear well before individuals develop full-blown obesity. Indeed, there is an important decline in renal plasma flow with increasing body mass within a range of values excluding overt obesity (BMI > 30 kg/m2) [28]. This decline is not paralleled by simultaneous down-sloping of the GFR and, as a consequence, filtration fraction (the ratio of these two measurements) increases linearly with higher BMI values. Thus the presence of obesity in almost half of the subjects [48.79%] may have contributed to the high prevalence of CKD in this study. The eGFR did not correlate with either systolic or diastolic blood pressure but those with stage II hypertension had a higher prevalence of CKD and because over half of the subjects had grade II hypertension rising blood pressure may result in rapid worsening of kidney function with worsening CKD.The various age categories showed significant variation in the prevalence of CKD, with those older than 60years having a prevalence of 50% while those older 80yrs had 100% prevalence and the difference between the groups were statistically significant. Thus increasing age is associated with decline in renal function as a result of a fall in GFR is due to reductions in the glomerular capillary plasma flow rate, and the glomerular capillary ultrafiltration coefficient. In addition, a primary reduction in afferent arteriolar resistance is associated with an increase in glomerular capillary hydraulic pressure. These hemodynamic changes occur in concert with structural changes, including loss of renal mass; hyalinization of afferent arterioles and in some cases, development of efferent glomerular arterioles; an increase in the percentage of sclerotic glomeruli and tubulointerstitial fibrosis. [29]. Our study showed that 80% of subjects had hyperuricemia, a very high prevalence and signifies a high cardiovascular risk in the presence of hypertension and CKD. This is because increased serum uric acid is an established risk factor for the development of CKD [30, 31] and recent studies shows that uric acid is independently associated with CKD and this effect may be medicated through hypertension [32], thus a high prevalence of hyperuricemia in untreated hypertensives means these treatment naïve hypertensives have excessively high cardiovascular risk.
5. Conclusions
- Undiagnosed chronic kidney disease is highly prevalent among untreated hypertensive patients seen in Port Harcourt. There is need for physicians to monitor serum creatinine, calculate eGFR routinely and evaluate for other cardiovascular risk factors as means of identifying subjects with kidney disease early to as to institute early measures to retard progression to end stage renal disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML