-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Practice
p-ISSN: 2326-1463 e-ISSN: 2326-1471
2017; 6(1): 1-8
doi:10.5923/j.cp.20170601.01

The Effects of Neurocognitive and Physical Tasks on End-Tidal Carbon Dioxide Levels and Respiratory Rate in Healthy Individuals - A Pilot Study
Patrick Siedlecki 1, Paolo Sanzo 1, 2, Carlos Zerpa 1
1School of Kinesiology, Lakehead University, Thunder Bay, Canada
2Northern Ontario School of Medicine, Lakehead University, Thunder Bay, Canada
Correspondence to: Paolo Sanzo , School of Kinesiology, Lakehead University, Thunder Bay, Canada.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective: A significant proportion of the Canadian population experiences breathing related health problems that may impact day-to-day living. Individuals with post-concussion syndrome may also report deficits in neurocognitive and physical abilities. Recent findings may suggest breathing to be linked to deficits commonly observed in this population. Research exploring breathing patterns within a concussed population, however, is relatively novel. The purpose of this pilot study was to explore the effects of completing a neurocognitive test versus a physical task on end-tidal carbon dioxide (ETCO2) and respiratory rate in a healthy population. Subjects: Eleven healthy participants (7 males, 4 females; age M=23.7 years; SD=4.9) were recruited to participate in the pilot study. Methods: Participants had their ETCO2 and respiratory rate recorded before and during a neurocognitive and physical task in a single 45-60 minute session. General demographic information was collected in addition to anthropomorphic measures (i.e., weight and height). The Nijmegen Questionnaire was also completed to assess breathing pattern. Baseline breathing data was then measured at rest in a sitting position before and during the completion of neurocognitive testing using the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT) battery. Baseline breathing data was then measured at rest in a standing position before and during four walking trials that varied in walking speed and treadmill elevation. There were also two minute breaks between each task and trial. Data were analyzed using Dependent Samples T-Tests and One-way Repeated Measures ANOVAs. The rejection criteria were at an alpha level p<.05. Results: Statistically significant increases were observed in respiratory rate (t(10)=-5.52, p=.001) during the ImPACT; ETCO2 (F(4,7)=14.18, p=.002) during all four walking trials; and respiratory rate (F(4,7)=7.02, p=.01) during three walking trials (i.e., slow self-selected walking speed at 5% grade, slow self-selected walking speed at -5% grade, and fast self-selected walking speed at 0% grade). No significant changes were observed in ETCO2 during the ImPACT battery or in respiratory rate during a slow self-selected walking speed at 0% grade. Conclusion: The current pilot study suggests that neurocognitive and physical tasks may result in changes in the ETCO2 and respiratory rate in a healthy population. These changes may be due to cognitive loading and increased physical demands on the cardiorespiratory system. Future investigations are warranted in examining the effects of completing similar neurocognitive and physical tasks in a concussed population.
Keywords: Respiration, Concussion, Physical task, Neurocognitive task
Cite this paper: Patrick Siedlecki , Paolo Sanzo , Carlos Zerpa , The Effects of Neurocognitive and Physical Tasks on End-Tidal Carbon Dioxide Levels and Respiratory Rate in Healthy Individuals - A Pilot Study, Clinical Practice, Vol. 6 No. 1, 2017, pp. 1-8. doi: 10.5923/j.cp.20170601.01.
Article Outline
1. Introduction
- Concussion can be defined as a temporary disturbance of brain function that is the result of a linear and/or rotational force being transmitted to the head causing a complex pathophysiological process to occur [1]. It was reported that over 94,000 Canadians, between the years of 2009 and 2010, sustained a concussion or another comparable brain injury [2]. It was also reported that, amongst individuals who suffered from a concussion, approximately 76% of them were under the age of 35 years [3]. These individuals may experience a wide variety of signs and symptoms that may be cognitive or physical in nature [4]. Pardini et al. (2004) identified four core symptom dimensions associated with concussion [5]. These were described as emotional symptoms (i.e., sadness, nervousness, and irritability), cognitive symptoms (i.e., attention problems and fatigue), somatic symptoms (i.e., visual problems, dizziness, headaches, and nausea), and sleep disturbances (i.e., insomnia). Fortunately, most individuals will completely recover within 1-2 weeks following a concussion; however, approximately 10-20% of the concussed population will continue to be symptomatic for weeks or months post-injury [1]. When this occurs, a concussion may sequela into what is known as post-concussion syndrome (PCS). Due to the variability of symptomatology, prescribing proper treatment for PCS may be difficult.There is no single treatment for PCS because the injury has variable symptomatology that affects individuals in different ways. Therefore, treatments must be selected based on subjective clinical complaints and objective observations. Of these signs and symptoms, abnormal breathing physiology and dysfunctional breathing patterns have not been thoroughly researched in the concussed population.A recent study found that female athletes with PCS had elevated end-tidal carbon dioxide (ETCO2) levels during submaximal aerobic exercise compared to a healthy control [6]. End-tidal carbon dioxide is the peak amount of carbon dioxide (CO2) found at the end of an exhaled breathe and represents partial pressure CO2 (PCO2) [7]. The elevated ETCO2 levels may have also contributed to aggravated symptoms and inhibited physical functioning (i.e., reduced maximum intensity and duration of workload). However, there is no current literature that has explored the effects that other tasks (i.e., rest versus the performance of a physical or cognitive task) have on breathing.Abnormal breathing physiology and dysfunctional breathing pattern can play a pivotal role in negatively affecting an individual’s life. In 2007, the Public Health Agency of Canada reported that over 11% of the country’s population has at least one of the top five chronic respiratory diseases (i.e., asthma, chronic obstructive pulmonary disease, lung cancer, tuberculosis, or cystic fibrosis) [8]. Similarly, the World Health Organization stated that a large proportion of the world’s population also suffered from asthma (300 million people), chronic obstructive pulmonary disease (210 million people), allergic rhinitis (400 million people), sleep apnea (100 million people), and other respiratory disorders (50 million people) [9]. These disorders may affect the quality of breathing and may result in altered arterial partial pressure CO2 (PaCO2), ETCO2, and respiratory rate levels. Arterial partial pressure CO2 is the balance of CO2 production and elimination in arterial blood vessels and is similar to ETCO2 [10]. Measuring these variables may assist in diagnosing or monitoring the disorder. With the vast amount of individuals who have a respiratory disorder, the addition of also sustaining a concussion in this group, may hinder recovery time and contribute to persistent concussion-like symptoms.Neurocognitive deficits are typically observed in individuals with concussion [11]. Executive function is one component of neurocognitive ability that is used in most everyday activities as it is used in attention control, decision making, visual processing, and spatial awareness [12]. In addition to neurocognitive deficits, individuals may also experience physical and physiological deficits (i.e., balance, dizziness, and increased heart rate) weeks or months post-injury [13, 14]. It is important to monitor breathing because psychological stress can increase the activity in the brainstem, which is the location of high command respiratory centres [10], and the anterior cingulated cortex (located in the corpus callosum), which is responsible for executive function [15]. Damage to the neural tissue surrounding these cerebral regions may affect breathing pattern. Chronic respiratory disorders may have similar deficits to those who have sustained a concussion. Individuals with chronic obstructive pulmonary disorder (COPD), a heterogenic chronic disease affecting airflow [16], were reported to have normal PaCO2 and respiratory rate at rest [17, 18]. When patients were tasked with completing physical exercise, individuals had significantly reduced respiratory rates compared to patients with dyspnea. Furthermore, patients with more severe COPD had significantly lower cognitive scores compared to less severe cases of COPD [16]. Similar, Betan, Cirak, Varol, and Ulcar (2015) found that individuals with obstructive sleep apnea syndrome had elevated PaCO2 levels during rest [19]. If PaCO2 is below 34 mmHg, blood flow to the brain may be reduced by up to 20% and may further negatively affect other physical or cognitive processes [20]. Certain individuals with chronic respiratory diseases may experience cognitive and physical deficits due to abnormal breathing. Therefore, if any of these individuals were to also present with a concussion simultaneously, the combination of the two disorders may inhibit or prolong recovery time even more.Therefore, the purpose of this study was to investigate the effects of completing a neurocognitive test using the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT) battery versus a physical task such as walking on a treadmill under different walking conditions (slow versus fast and uphill versus downhill) on measures of ETCO2 and respiratory rate in a healthy population.The study was guided by the following questions: (1) Is there a difference in ETCO2 measured when sitting in a resting position compared to after completing the ImPACT battery?; (2) Is there a difference in respiratory rate measured when sitting in a resting position compared to after completing the ImPACT battery?; (3) Is there a difference in ETCO2 measured when standing in a resting position compared to when walking at different speeds (slow versus fast walking) and elevations (uphill versus downhill)?; and (4) Is there a difference in respiratory rate measured when standing in a resting position compared to when walking at different speeds (slow versus fast walking) and elevations (uphill versus downhill)? This was also an exploratory study investigating the feasibility of using a similar methodology in the future within a concussed population.
2. Methods
- After obtaining ethical approval from the academic institutional Research Ethics Board, the effects of completing a neurocognitive task as compared to a physical task on breathing physiology was examined in prospective healthy individuals.
2.1. Participants
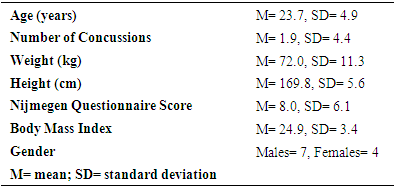
- Eleven participants (7 male and 4 females), between the ages of 18-32 years (M = 23.7 years, SD = 4.9), were recruited through convenience sampling. Inclusion criteria required participants to be between the ages of 18-35 years to reduce the effects of puberty on ETCO2 [7] and limit the effects of aging on breathing (i.e., decreased lung function) [21]. Participants were excluded if they had a physical injury (i.e., lower limb fracture, muscle strain, or ligament sprain), a neurological disorder (i.e., stroke, cerebral palsy, or Down’s Syndrome), or a respiratory disease (i.e., asthma or influenza) that may have affected their ability to safely complete a walking or cognitive task.
2.2. Outcome Measures and Instrumentation
- A demographic interview was conducted to gain general demographic information about the participant’s name, year of birth, age, and medical history regarding neurological and/or respiratory diseases/disorders. Additionally, objective measurements of the participant’s height (cm) and weight (kg) were also measured and recorded. Following the demographic interview process, the Nijmegen Questionnaire (NQ), ImPACT battery, and CapnoTrainer were also used throughout the study.
2.2.1. Nijmegen Questionnaire
- The NQ was completed by participants as a screening tool to identify his/her breathing pattern (i.e., hyperventilation, apnea, or bradypnea). The NQ is a 16-item self-report questionnaire that assesses different physiological systems such as the cardiovascular, neurological, and gastro-intestinal systems, and the psychological processes that have been known to influence or alter breathing patterns [22]. These questions were rated on a 5-point Likert Scale ranging from 0 (never) to 4 (very often) and higher scores highlighted the likelihood that individuals may have a breathing disorder (maximum score possible is 64). Participants were allowed to continue participating in the study if his/her scores fell between the questionnaire’s normal range (0-23). Van Dixhoom and Duivenvoorden (1985) reported that the questionnaire had a sensitivity of 91% and specificity of 95% in identifying hyperventilation syndrome [23]. The NQ is an easy to use questionnaire that does not require trained specialists to administer, record, and interpret the results, making the questionnaire ideal for identifying abnormal breathing patterns [24].
2.2.2. Immediate Post-Concussion Assessment and Cognitive Test
- The ImPACT is a computerized neurocognitive battery designed to measure baseline executive function and identify deficits in neurocognitive ability in healthy and concussed populations (ImPACT Applications, Inc.). The ImPACT assesses six specific dimensions of executive function in a 20-30 minute assessment. Visual and verbal memory, working memory, processing speed, visual motor skills, and reaction time are assessed individually or in combination with other dimensions of executive function. The battery itself consists of three sections: demographic information, the Post-Concussion Symptom Scale (PCSS) questionnaire, and six test modules.The Demographic Information section records general anthropometric characteristics similar to that obtained in the previously completed interview. The PCSS questionnaire is a 19-item self-reported questionnaire that ranks concussion symptoms on a 6-point Likert Scale ranging from 1 (minor) to 6 (severe) and is embedded within the battery. The final section of the ImPACT contains six modules measuring executive function [25]. The modules included are Word Memory, Design Memory, X’s and O’s, Symbol Match, Color Match, and Three Letters. Each module assesses the ability to complete a specific or a combination of different types of executive function and constructs a composite score.The ImPACT has been found to discriminate between individuals with and without a concussion [26]. In addition, the battery has also been found to identify slight deficits and changes in neurocognitive function in individuals who do not appear to have any deficits at the time of assessment [27]. In comparison to pen and paper neurocognitive tests, the ImPACT is less prone to practice effects. Furthermore, the ImPACT is more cost and time-effective and does not require training in order to interpret results as compared to pen and paper tests [1]. Therefore, the test battery can accurately replicate a neurocognitive task.
2.2.3. CapnoTrainer©
- The CapnoTrainer© is a portable capnography breath analyser used to measure ETCO2 and respiratory rate (Better Physiology, Santa Fe, NM). Data is collected and analysed with computerized software (CapnoLearning 3.0 PhysioLab) in the form of capnogram waveforms. McLaughlin, Goldsmith, and Coleman (2010) monitored depressed levels of ETCO2 in 29 participants with chronic lower back and neck pain using the CapnoTrainer© [28]. Using a built-in breathing retraining program with the participants, ETCO2 levels returned to a normal range (35-40mmHg) which also improved the number and severity of subjective complaints, perceived pain, and the ability to perform symptom limited activities.Furthermore, the device was found to be statistically reliable during quiet breathing and mental activities (i.e., quiet breathing, mental mathematics, and controlled breathing exercises). In addition, the device also had moderate (.68) to strong (.79) convergent validity when measuring ETCO2 and respiratory rate compared to another open circuit spirometer [29]. Moreover, the CapnoTrainer© is a cheaper, non-invasive, portable, easy to use, and time efficient method of measuring ETCO2 compared to standard capnography breath analysers found in hospital critical care units [7, 30].
2.3. Procedure
- Participants completed a single, 45-60 minute test session. All testing was completed in a quiet laboratory setting within the academic institution. General demographic information on the participant was recorded using the demographic interview (i.e., name, year of birth, age, history of neurological or respiratory disorders). The participant’s weight and height was also measured and recorded during this process. Following the demographic interview, the NQ was completed to ensure the participant’s breathing fell within the normal breathing pattern (total score ranging from 0-23). Following completion of the NQ, participants were fitted with a nasal cannula which was connected to the CapnoTrainer©. Data from this device was collected throughout the entire test session. Participants were also instructed to breathe through their nose and refrain from talking during the tasks.During the neurocognitive task, baseline ETCO2 (mmHg) and respiratory rate (breaths/min) were collected for 40 seconds (only the last 20 seconds of the baseline data was used in the statistical analysis) during quiet breathing while resting in a sitting position. Next, the ImPACT was completed on a laptop computer with a wireless mouse to assess the participant’s neurocognitive ability and simulate a neurocognitive task. The participant was instructed to raise his/her hand when beginning a new module to track progression through the test. After completing the ImPACT, a two minute recovery period occurred to allow for the breathing pattern to return to baseline values.During the physical task, baseline breathing data was also collected for 40 seconds during quiet breathing while resting in a standing up-right position. There were four different walking trials completed that varied on walking speed and treadmill elevation. Each walking trial lasted three minutes (only the last 60 seconds of the data were used in the statistical analysis) and was followed by a two minute rest period. During the rest periods, the treadmill came to a complete stop and participants rested on the treadmill in a standing position. The first three walking trials consisted of walking at a slow self-selected speed between 3-4 mph with the treadmill elevated to a 0% (T1), 5% (T2), or -5% grade (T3). The fourth trial was a fast self-selected speed between 4.5-5.5 mph with the treadmill set to a 0% grade (T4).
2.4. Statistical Analysis
- Descriptive statistics were used to compare the mean and standard deviations for individual ETCO2 and respiratory rate data. This data was collected through the CapnoTrainer© and transferred into Microsoft’s Excel computerized program for initial analysis. Data analysis was then completed using IBM SPSS 24 for statistical analysis. Statistical significance was determined with an alpha level of p<.05. Two dependent samples T-tests were conducted to determine if significant differences existed between pre- and post-neurocognitive tasks on ETCO2 or respiratory rate. One-way repeated measures ANOVAs were also conducted to determine if significant differences existed between the baseline and the four walking trials on measures of ETCO2 and respiratory rates. If a statistical significance was found, a Bonferroni post-hoc analysis was performed to determine pair mean comparisons between tasks.
3. Results
- Eleven participants were included in the study. The participants’ anthropometric characteristics are summarized in Table 1.
|
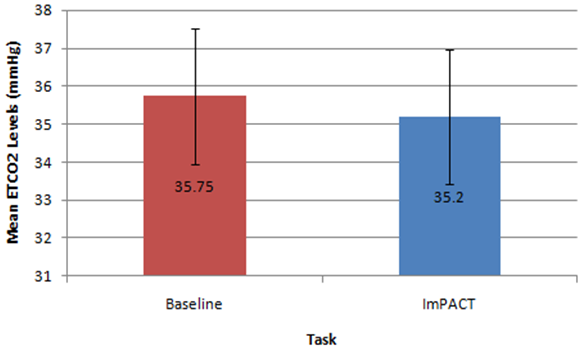 | Figure 1. Mean data for ETCO2 (mmHg) levels measured at baseline versus during the completion of the ImPACT test battery |
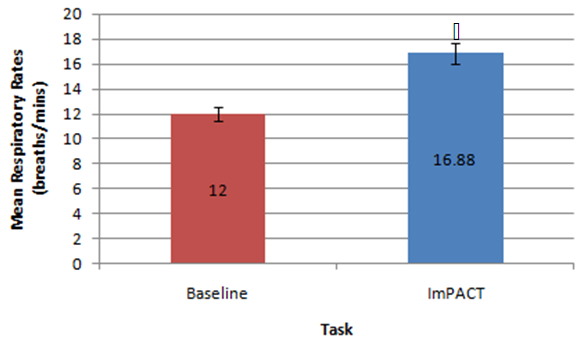 | Figure 2. Mean data for respiratory rate (breaths/minute) measured at baseline versus during the completion of the ImPACT test battery.  indicates statistical significance indicates statistical significance |
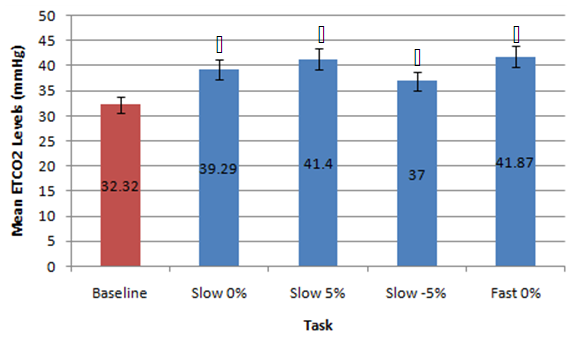 | Figure 3. Mean data for ETCO2 (mmHg) levels measured during baseline versus different walking conditions. 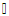 indicates statistical significance indicates statistical significance |
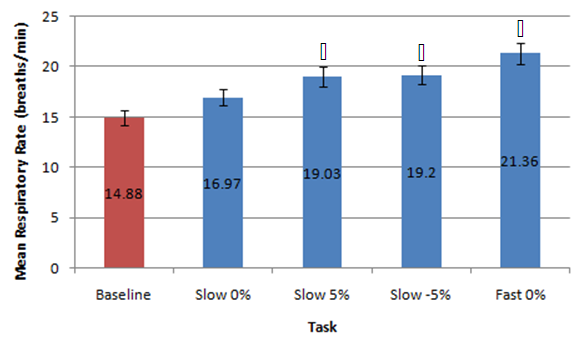 | Figure 4. Mean data for respiratory rate (breaths/minute) measured at baseline versus different walking conditions. 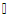 indicates statistical significance indicates statistical significance |
4. Discussion
- This preliminary study investigated the effects of the completion of neurocognitive and physical tasks on ETCO2 and respiratory rate in healthy individuals. It was found that completing neurocognitive tasks (ImPACT testing battery) did not affect ETCO2 levels compared to baseline resting values measured in a sitting position. Although the mean ETCO2 level during the ImPACT was slightly lower compared to baseline, ETCO2 levels at rest in a sitting position fell between the average healthy range (35-40 mmHg) [31]. This finding is surprising, as previously reported findings in a healthy population suggested ETCO2 decreased with the completion of cognitive tasks [15]. A meta-analysis examining the effects of cognitive loading on respiration stated that ETCO2 was overwhelmingly found in the previous literature to decrease due to the stress that a cognitive task placed on an individual [15]. Kelley et al. (1992) found that cognitive tasks (i.e., mental mathematics or playing video games) may increase the blood flow velocity of specific cerebral arteries (i.e., middle cerebral artery), which may also increase the PCO2 in local regions of the brain [32]. The central nervous system maintains PCO2 through auto-regulation to maintain homeostasis at rest and uses respiration as one of the methods to adapt physiological processes to changing environmental demands [15, 33]. Negatively affected cognitive scores have been found in healthy individuals with abnormal breathing pattern [34]. However, all participants in the current study completed the ImPACT test battery and scored in the 50th percentile or above and all participants had normal NQ scores; therefore, it is not expected that participants had dysfunctional breathing patterns that contributed to this finding. Moreover, Lee et al. (2015) reported that participants performing a dual-task (walking while completing a cognitive task such as playing Tetris or a paced auditory serial addition task), did not experience anymore symptoms than a group that was only walking [12]. None of the participants in the current study self-reported any symptoms during or after completing the ImPACT battery. Finally, since cognitive tasks may stimulate a certain amount of stress, the lack of change in ETCO2 may have occurred because participants were familiar with the test and did not consider the test challenging.Conversely, the methodology used and study data showed that completing the ImPACT battery did significantly increase respiratory rate. The baseline resting respiratory rate values taken in a sitting position were also found to fall within the normal resting range (8-14 breaths per minute) [7]. This finding was expected as respiratory rate increases with cognitive demands. Grassmann et al. (2016) reported that, not only did respiratory rate increase but tasks that were perceived as being more challenging had higher respiratory rates [15]. It was also stated that over 48% of studies monitoring respiratory rate during cognitive tasks found medium to high effects for increased respiratory rate. It was found that there was a significant difference between ETCO2 values measured at rest in a standing position and during different walking conditions. These findings are supported by previous findings that reported ETCO2 increases with physical exertion [6]. In the current study, ETCO2 was observed to increase when walking speed increased and also when the treadmill deviated from a 0% grade. An inclined treadmill reproduced a greater increase in ETCO2, as opposed to a declined treadmill elevation. The mean ETCO2 value at rest in a standing position was below that of normal resting ETCO2 range. This may be due to participants being introduced to a new position (standing on top of a treadmill) and explaining the procedure of the new task/trials. This sudden change in situation and task may have resulted in increased anxiety and stress that would, in turn, decrease ETCO2 [35]. The significant differences observed in the current study data may have been less significant or nonexistent if ETCO2 values fell within the normal range at rest. Bennett and Fordcye (1993) found that PaCO2 levels were close to or at resting levels during mild and moderate intensity aerobic exercise [36]. In addition, PaCO2 increased slightly as exercise intensity increased up to 75% of an individual’s maximum workload capacity and decreased once the lactate threshold was surpassed [37]. Similarly, ETCO2 was found to increase during walking on a treadmill at a mild intensity and peaked during moderate speeds before decreasing with faster, more challenging speeds [6]. This can be explained by the fact that when glucose is converted into lactate during glycolysis (one of the processes that create energy), CO2 is produced as a by-product and must be eliminated through respiration. However, once the lactate threshold is passed, the body cannot eliminate CO2 as quickly as it is being produced. Therefore, CO2 accumulates in the blood and ETCO2 levels decrease.Lastly, there was a significant difference in respiratory rate in three of the four walking trials compared to respiratory rate measured when resting in a standing position. Respiratory rate was found to be at the upper boundary of a normal respiratory rate range. As walking speed increased and the elevation on the treadmill deviated from a 0% grade, respiratory rate increased. Unlike ETCO2, respiratory rate progressively increased with the different walking trials. Respiratory rate has a positive relationship with the intensity of physical exercise. During lower exercise intensities, respiratory rates steadily increase at a linear rate [36]. Furthermore, once the lactate threshold is reached, respiratory rate begins to increase disproportionately to the amount of CO2 that is produced [10]. The rapid increase in respiratory rate occurs because the brain attributes high levels of CO2 with low levels of oxygen [38]. Consequently, oxygen demand is exaggerated and respiratory rate further increases. Therefore, as energy demands in muscles rises, respiratory rate must be increased to supply muscles with oxygen. This can explain why there was an increase, but not a significant difference, in respiratory rate between rest in a standing position and T1. The small increase in respiratory rate at lower intensities is due to oxygen demands being met by increased tidal volume (amount of oxygen in a single breath) rather the body solely relying on more breaths. Some limitations of the current study include the small sample size reducing the strength of the inferences that can be made. Secondly, the variable nature of breathing patterns and ETCO2 also makes it difficult to reproduce accurate data. A cough, laugh, sudden loud noise, bending over, and quiet reading may all affect ETCO2. Thirdly, the self-selected walking speed ranges may have added additional variability to the data as choosing speeds on either end of the spectrum may have changed the ETCO2 and respiratory rates. Future research should consider measuring breathing physiology in a concussed population, as auto-regulation may affect respiration. Comparing male and female breathing patterns could establish a difference between genders. This may occur because females are more likely to be susceptible to hormonal changes, which has also been found to affect breathing [7]. The ability to analyse more specific intensities of physical exercise may provide a better description of how breathing physiology is affected by exact exercise intensities. Also, looking at different types of executive function may identify specific changes to breathing.
5. Conclusions
- This study showed that the completion of neurocognitive and physical tasks can affect breathing physiology in a healthy population. Specifically, tasks that involved executive function (i.e., processing speed, concentration, or working memory), can create cognitive stress that may affect respiration. Although ETCO2 did not change during the ImPACT test battery compared to baseline, there were no signs that would suggest that participants had abnormal breathing patterns. The ImPACT test battery was also successful in replicating past literature findings as respiratory rate increased during the completion of the ImPACT. Breathing physiology may also be affected during less demanding physical activities, such as walking at a slow or fast speed. This study was able to simulate mild and moderate exercise intensity as ETCO2 elevated compared to baseline in all walking trials. Additionally, respiratory rate increased throughout each walking trial in a positive linear relationship. Therefore, walking at different speeds may be useful in simulating varying levels of difficulty in a physical task. This study revealed that normal changes in breathing physiology during neurocognitive and physical tasks, within healthy individuals, do not negatively impact performance. However, due to the potential deficit in auto-regulatory function in certain concussed individuals, the brain may not be able to adapt to changes in the environment (cognitive versus physical stress). Therefore, observing the effects of a similar protocol on breathing physiology may prove to be beneficial in establishing a new form of treatment for neurologically impaired and concussed populations.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML