-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Practice
p-ISSN: 2326-1463 e-ISSN: 2326-1471
2016; 5(1): 6-15
doi:10.5923/j.cp.20160501.02

The Usefulness of a Carpal Tunnel Compression Assessment Tool: Evidence of Reliability and Validity in Assessing Carpal Tunnel Syndrome
Devin Jackson , Carlos Zerpa , Daniel Vasiliu , Paolo Sanzo , Ian Newhouse
School of Kinesiology, Lakehead University, Thunder Bay, Canada
Correspondence to: Carlos Zerpa , School of Kinesiology, Lakehead University, Thunder Bay, Canada.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Carpal Tunnel Syndrome (CTS) affects many individuals throughout their daily lives and is the result of compression of the median nerve in the carpal tunnel space. At present, there is a lack of CTS assessment tools that are easy, accurate, and less expensive to implement than traditional methods to help healthcare professionals identify individuals with CTS. The purpose of this study was to provide evidence for the validation of a simple and easy to implement carpal tunnel compression assessment tool (CTCAT) to more accurately assess CTS. The CTCAT is a portable testing device that can easily be used by medical professionals to assist with reducing diagnostic wait times and/or costs associated with CTS. As the CTCAT is a new instrument, much of the work in this study involved assessing the reliability and validity of this prototype development and a 10-point likert pain scale. A sample of 19 participants was used for this study (10 in CTS group, 9 in control group). Physical testing was completed with the CTCAT based on the Carpal Compression Test protocol. Construct-related evidence of validity was provided by relating the CTCAT to the Boston Carpal Tunnel Questionnaire and a 10-point Likert pain scale. The findings were analyzed by using correlation techniques (i.e., intraclass and Pearson’s correlations) and measures of internal consistency (i.e., Cronbach’s alpha). The outcome of this study revealed that the CTCAT produced reliable and valid measures when assessing participants with CTS based on response time to symptom onset on two testing dates. This outcome may have implications for clinicians because it provides an avenue to use a simple, reliable and valid instrument to monitor, assess and prevent CTS.
Keywords: Carpal tunnel syndrome, Carpal compression test, Assessment tool, Validity
Cite this paper: Devin Jackson , Carlos Zerpa , Daniel Vasiliu , Paolo Sanzo , Ian Newhouse , The Usefulness of a Carpal Tunnel Compression Assessment Tool: Evidence of Reliability and Validity in Assessing Carpal Tunnel Syndrome, Clinical Practice, Vol. 5 No. 1, 2016, pp. 6-15. doi: 10.5923/j.cp.20160501.02.
Article Outline
1. Introduction
- Carpal tunnel syndrome (CTS) is a median nerve entrapment neuropathy. Symptoms may vary from numbness, tingling, or burning in the median nerve distribution spanning the volar and first three and a half digits, to hand weakness, and functional disturbances [4, 25]. As the condition worsens, the motor supply of the median nerve is often more affected, leading to general clumsiness in the hands for fine and gross motor tasks and in the overall manual dexterity. When the motor control of the hand becomes affected, CTS is considered to be in the advanced stages [20].Females are more commonly affected with CTS than males at a staggering rate of four to one after the age of 40, which may be mainly due to the smaller wrist size of females [20]. The incidence rate between the two sexes, however, is nearly equal before the age of 40 years [20]. The carpal tunnel space of females is smaller than that of males but has the same number and size of tendons and nerves passing through it, leading to an increased chance for aggravation to occur [20]. After the age of 40, with hormonal changes in a woman’s body, fluid retention and general swelling can lead to an increased incidence of CTS in women [28]. Another factor that has contributed to the increased prevalence amongst women is the fact that in the past, women filled the majority of clerical positions such as secretarial, clerical, accounting, and bank teller positions. These mainly hand-wrist dominant careers were also a strong contributor to the development of CTS in the female population [20]. Carpal tunnel syndrome is high for individuals working in both sedentary (i.e., desk) and active jobs (i.e., manual labour) [27]. Carpal tunnel syndrome is also commonly associated with other disorders such as rheumatoid arthritis, diabetes, and hypothyroidism; in individuals receiving estrogen replacement therapy [29]; or who have undergone an oophorectomy [22]; and in pregnant females [31]. Therefore, in the assessment process these other comorbidities must be identified as well and treated appropriately when necessary by healthcare providers. Carpal tunnel syndrome symptoms can also be caused by heavy manual work, exposure to vibrations, and awkward positions of the wrist [24] and [27] and [32]. Manktelow, Binhammer, Tomat, Bril, and Szalai [21] found that 50% of the reported CTS cases in the province of Ontario in 1996 (n=964) originated from fabrication or assembly line workers. Clerical workers accounted for 16% of the diagnosed population, with up to 75% of all cases reported having bilateral involvement. Manktelow et al. [21] also reported that in some cases, patients experienced symptoms for as long as three years before receiving treatment; with as much as 46% of patients reporting ongoing symptoms even after receiving treatment. With so many cases reported, and with such a high rate of recurrence, there is an increasing need for better diagnostic tools and methods.Although CTS is mainly an overuse injury where the onset of symptoms cannot be attributed to a single event, in some cases, it may be brought on by repetitive use combined with episodes of tissue damage to the carpal tunnel caused by injuries sustained during a fall onto an outstretched hand [6]. The clinical presentation and manifestation may be either unilateral or bilateral. It is well documented that if the condition is unilateral, it often appears in the dominant wrist. Similarly, if the condition is bilateral, the symptom severity tends to be greatest in the dominant wrist [20]. The treatment of CTS may include a variety of options ranging from conservative measures to surgical. Various conservative, pharmacological, and surgical release methods have been proposed with varying responses [12]. Optimally, the least invasive treatment methods should be utilized and exhausted first. As Love [20] stated, the most essential care is through the early detection of symptoms, but before a patient seeks surgery as a treatment option, he/she should seek advice from a professional specializing in upper quadrant, wrist, and hand dysfunction.Overall, the high incidence of this disorder and available treatment options for CTS is costly to the healthcare system, [18, 34]. Given the high incidence of CTS and the associated cost in lost productivity and treatment ranging from $12,500 to $28,000 per individual, research that may aid in the early and accurate assessment, identification, and diagnosis of CTS is needed.One way to increase the diagnostic efficacy for CTS is through electrodiagnostic testing, such as electromyography (EMG) and nerve conduction studies [2]. Of the two, NCS is more valuable than EMG testing in CTS diagnoses due to the demyelination process involved in CTS with NCS better assessing the demyelination of the nerve tissue in comparison to EMG [33]. Since electrodiagnostic techniques need to be administered under strict conditions to gain the best insight into the patient’s condition, variables such as electrode placement, distance measurements, stimulation intensity, and skin temperature must be closely monitored for the most reliable results [33]. Although this testing method produces reliable results, the procedure is typically expensive [11].Currently, there are several CTS self-report questionnaires and few clinical tests that are accurate and quick and easy to implement to obtain both subjective and objective information related to the CTS. Many of the questionnaires, however, do not inquire directly about the hand and wrist, and may lead to other improper assessments and incorrect diagnoses [14]. In addition, CTS self-report questionnaires contain a certain degree of biased information due to the subjective nature of the assessment tools. There are currently several proposed questionnaires used to assess and diagnose CTS. The Boston Carpal Tunnel Questionnaire (BCTQ), created by Levine et al. [19], is one of the most commonly used outcome measures in the assessment of patients with CTS [26]. The focus of the questionnaire is on two scales, the symptom severity scale (SSS, 11 items) and functional status scale (FSS, 8 items). The scales have been reported to be highly reproducible, showing intra-class reliability of r= .91 for the SSS and an even higher r= .93 for the FSS. Cronbach’s alpha was found to be .89 for the SSS and .91 for the FSS [26].The most common clinical tests used in the diagnosis of CTS include the Carpal Compression Test (CCT), Phalen’s Test, and the Tinel Test, all of which attempt to reproduce the patient’s symptoms in order to confirm the diagnosis. The Phalen’s test is conducted by asking the patient to hold his or her wrist in a forced flexion for 30 seconds. The Tinel test is conducted by slightly tapping over the never that produces a tingling sensation. The Carpal Compression Test developed by Durkan [7] is completed by applying a steady pressure of 150mm Hg directly over the carpal tunnel space with the pads of the thumb tips in order to compress the median nerve. All three tests are provocative in nature, but may give false negative findings if the patient has excessive median nerve dysfunction, lack sensation in the region, or if the disease is in the later stages [9].The CCT, however, has been found to be more sensitive and specific than the Tinel and Phalen’s Tests in clinical assessments. When administered to participants with CTS, 87% of the participants tested positive with the CCT, 69% tested positive with Phalen’s Test and 56% tested positive with the Tinel Test [7]. Overall, the evidence on the utility of the Tinel Test and and Phalen Test is highly variable with some studies indicating that these tests are not helpful in diagnosing CTS [5]. The issue that arises with the diagnosis of CTS is that there is not one specific test that is used. Medical professionals all have his/her own preferred method for testing patients complaining of wrist pain and numbness. As Falkiner et al., [9] stated, there is no standardized diagnostic technique for CTS. None of the physical tests, questionnaires, or electrodiagnostic techniques is, by itself, a “gold standard” for diagnosing CTS. In order to implement less expensive CTS treatment methods, health professionals and employers must use CTS assessment tool measures that are simple to use, but with a high degree of validated evidence. That is “the degree to which evidence and theory support the interpretation of test scores” (p.9) [30]. This degree of validated evidence may include: construct-related evidence, content-related evidence, and concurrent-related evidence.While validity is concerned with providing evidence to support inferences made from the assessment results of the tool or device, reliability is concerned solely with how test scores are expected to vary when repeatedly using the assessment tool [13]. That is, evidence of reliability can be provided for the use of CTS assessment tools by examining the internal consistency of the items in relation to a latent variable or construct (e.g., pain) [3, 15]. Another approach is by implementing a pre- and post-test design to examine the reliability of the CTS test measures. The strength of the relationship between the pre- and post-test measures can be used as an indicator of the variance present when repeating the test [3]. Based on the lack of accuracy and simplicity of current CTS assessment tools, there is a need to develop new assessment tools with a high degree of reliability and validity that are easy to use and administer in CTS diagnostics to minimize measurement error, healthcare cost, and time. With these concerns in mind, the main purpose of this study was to to provide evidence of reliability and validity for the use of a new carpal tunnel compression assessment tool (CTCAT) prototype by comparing it to the BCTQ and 10-point self-report Likert pain scale. Before addressing the main purpose of the study, the researchers verify the reliability of the BCTQ, which was used as a standard to assess measures of pain for healthy and CTS participants. The researchers also provided evidence of reliability and validity for the use of the 10-point likert scale as criterion to assess pain during the administration of the carpal compression test (CCT) to further validate the CTCAT. Three research questions were developed to address the purpose of the study: 1) How reliable are the measures obtained from the BCTQ with the current data? 2) Can a 10-point self-report Likert pain scale question be used to assess CTS when compared to the BCTQ? 3) Is the CTCAT time to symptom onset a reliable and valid measure to assess CTS when compared to the BCTQ and 10-point self-report Likert pain scale?
2. Methods
2.1. Participants
- Nineteen participants over the age of 18 partook in this study. Out of the 19 participants, 10 had CTS and 9 were healthy. Initially, this study aimed to include 40-50 participants with CTS and a large number of medical clinics were approach, but unfortunately some of these clinics were not frequently treating patients with CTS and as a result, the researchers were only able to recruit 10 participants with CTS after 3 months of recruitment. All potential participants recruited for this study were over the age of 18 years.
2.2. Instruments
- The following instruments were used in the current study:2.2.1. Boston carpal tunnel questionnaire (BCTQ). This instrument was used as a measure of pain felt before the CTS test and to provide evidence of validation for the use of a 10-point self-report Likert scale and CTCAT. This instrument is composed of a symptom severity scale to assess pain/discomfort and a functional status scale to assess difficulty with certain activities of daily living. The BCTQ scales have been found to be highly reproducible with intra-class correlations of 0.89 for pain/discomfort scale and 0.91 for the functional status scale [26]. The limitation of using this instrument alone is that it only provides measures of pain felt before the CTS test and not during the CTS test. 2.2.2. 10-point self-report Likert scale. This instrument was developed and used by the researchers to compute the participant’s level of pain felt during the CTS test and provide evidence of validation for the use of the CTCAT. The level of pain felt during the test was computed by subtracting the pre-test pain data from the post-test pain data. 2.2.3. Carpal tunnel compression assessment tool (CTCAT). This instrument was developed and used by the researchers to mimic the CCT protocol developed by Durkan [7]. As depicted in Figure 1, the instrument contained a small box with a Velcro strap to place and secure the participant’s hand in the proper testing position. The instrument also contained a pressure ball placed on an inflatable bladder to push directly into the carpal tunnel space and compress the median nerve in the same fashion as health care providers would do when conducting a CCT. A hard ball was mounted on the instrument for the participant to rest the palm of the hand on during testing. The instrument minimized error during the CCT by removing the subjective judgment of health care providers as well as differences in health care providers’ thumb pad size during the CCT.
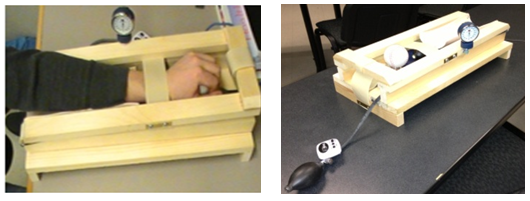 | Figure 1. Proprietary Carpal Compression Assessment Tool |
2.3. Procedures
- 2.3.1. Recruitment. Carpal tunnel syndrome participants were recruited by submitting recruitment packages multiple times to 10 large clinics (e.g., physiotherapy, chiropractic, medical doctor) in the community. Before distributing the recruitment packages to the medical clinics, practicing professionals were asked about his/her willingness to help recruit participants for the study. If the professional expressed that he/she was currently treating patients with CTS and willing to assist with recruiting participants, recruitment packages were left at their clinic to be distributed to the potential participants. Control participants, however, were recruited directly by the researchers using posters placed on bulletin boards at the academic institution. The age of participants included in this study ranged from 20 to 67 years. The CTS group included six female participants and four male participants, while the control group was composed of six female participants and three male participants. The mean age of the CTS group was 46 years, while the mean age of the control group was 34 years.2.3.2. Exclusion. Potential participants for the CTS testing group were excluded if his/her CTS symptoms and pain were not clinically diagnosed by a medical professional as truly being CTS. These individuals were excluded from the study to ensure that the testing was only completed on participants with CTS and not other hand and wrist ailments. Potential participants for the control group were excluded if they reported any hand and/or wrist difficulties such as numbness, soreness, or stiffness in the hand and wrist area.2.3.3. Testing. Participant testing took place in the academic institution research laboratory. Participant testing sessions took place after work hours, if required, to allow participants the flexibility to attend their testing sessions on the scheduled days at a time that was convenient to them. The testing period lasted a total of one month. Participant testing was approved by academic institutional Research Ethics Board before testing commenced.Participants were administered two physical testing sessions, two days apart, with each testing session containing a pre- and post-test to answer the research questions pertaining to this study. Both testing sessions that were carried out used the exact same testing procedures and instruments including the BCTQ, the 10-point Likert pain scale, and the CTCAT.Each participant (control group and CTS group) was asked to complete the consent form upon arriving on his/her first day of testing. Once the consent form was completed, the participant was asked to complete the BCTQ in regards to his/her CTS symptoms/discomfort over the previous 24 hour period. All participants were asked to complete the BCTQ as honestly as possible to avoid skewed testing results for each testing session. Although the BCTQ has strong evidence of reliability and validity as stated by Sambandam et al. [26], further evidence of reliability were provided with the current data to verify the consistency of the measures obtained from the BCTQ across replications with the current data. The participants (both CTS and non-CTS) were then asked to rate his/her CTS pre-test pain via the 10-point Likert scale before conducting the CCT on the CTCAT device during both testing sessions.To conduct the CCT, the participant was asked to insert his/her dominant hand into the CTCAT device with the forearm in a pronated position and the carpal tunnel area placed directly over the centre of the pressure ball as depicted in Figure 1. Once the wrist rested on the pressure ball in the proper position, the wrist angle was adjusted through the use of an adjustable ball rest. The pressure bladder was then inflated to 150mmHg. Pressure was maintained at 150mmHg for a maximum of 30 seconds based on testing protocol guidelines stated by Durkan [7]. Participants were reminded that if their CTS symptoms worsened during testing, to pull on the strap release mechanism to discharge the pressure applied on the carpal tunnel area and stop the testing immediately.After completing the CTCAT test, participants were asked again to rate his/her level of pain/discomfort using the 10-point Likert scale as a post-test pain measure. Lastly, before participants left the first testing session, a second testing session was booked two days later and the same testing procedures were administered.2.3.4. Analysis. Cronbach’s alpha inter-item reliability tests were conducted to verify the internal consistency of the BCTQ questionnaire items as measures of pain when diagnosing individuals with CTS. Intraclass correlations were also computed to verify the consistency across replications of the BCTQ by comparing the BCTQ scores between sessions one and two.Two intraclass correlational analyses were conducted to provide evidence of reliability for the use of the 10-point Likert scale. The first intraclass correlation compared the 10-point Likert scale pre-test pain measures between session one and two. The second intraclass correlation compared the 10-point Likert scale post-test pain measures between session one and two. A third intraclass correlation was also conducted to compare the pre-test 10-point Likert pain scale scores to the BCTQ part one scores for session one and two respectively to provide evidence of concurrent validity for the use of the 10-point Likert scale as a criterion to measure pain.Evidence of reliability for the use of the CTCAT was provided using the test-retest method. That is, intraclass correlations were used to compare the times measured between sessions one and two to examine the degree to which the time measures by the CTCAT were consistent across replications.To provide convergent-related evidence of validity for the use of the CTCAT, a Pearson’s moment correlation was used to compare the CTCAT time scores to the pain measured during the test. The pain measured during the test was computed by subtracting the 10-point Likert scale pre-test pain measures from the post-test pain measures.To provide discriminant-related evidence of validity for the use of the CTCAT, a Pearson’s moment correlation was used to compare the CTCAT time measures (time to symptom onset measures) to the BCTQ part one and two.
3. Results
- The results of this study provide evidence of reliability for the use of the BCTQ. The results also provide evidence of reliability and validity for the use of the 10-point Likert scale to assess pain during the administration of the CCT. Finally, the results of this study provide evidence of reliability and validity for the use of the CTCAT to address the main purpose of this study. To answer the research questions, descriptive, inferential statistics, and correlations were used as follow:
3.1. How Reliable are the Measures Obtained from the BCTQ with the Current Data?
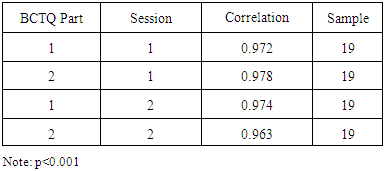
- This research question was formulated to verify the internal consistency of the questions included in part 1 and 2 of the BCTQ. Part 1was used to assess participant CTS pain and discomfort levels experienced within a 24 hour period before the CCT. Part two was used to assess difficulty with activities of daily living within a 24 hour period before administering the CCT. The results, as shown in Table 1, indicate strong internal consistency for the questions of the BCTQ addressing pain and difficulty for each administration of the BCTQ. This reliability evidence is based on high Cronbach’s alpha values obtained for session 1 and 2 as shown in Table 1.
|
3.2. Can a 10-Point Self-report Likert Pain Scale Question be used to assess CTS when compared to the BCTQ?
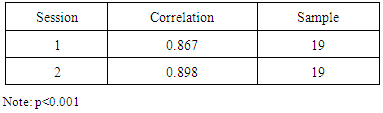
- This question was formulated to provide evidence of reliability and validity for the use of a 10-point self-report Likert scale as a measure of perceived pain during the administration of the CCT. This Likert scale was also used as a criterion for the validation of the CTCAT. To provide evidence of reliability, an intraclass correlation analysis was conducted by comparing the 10-point Likert scale pain measures obtained from session 1 and 2 including non-CTS and CTS participants. The results in Table 2, indicate strong significant correlations across replications of the 10-point likert scale measures suggesting consistency across replications of the pain measures felt by the participants during the administration of the CCT. This outcome provide evidence of reliability for the use of the 10-point Likert scale.
|
|
3.3. Is the CTCAT Time to Symptom Onset a Reliable and Valid Measure to assess CTS when compared to the BCTQ and 10-point Self-report Likert Pain Scale?
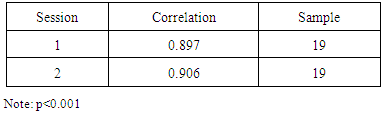
- To address this research question, only participant with CTS were included. Evidence of reliability was obtained by conducting an intra-class correlation to compare the CTCAT measures of time to symptom onset between sessions 1 and 2. The strong significant correlation, r= .906, p< 0.001, n= 10 suggested that it is possible to reproduce the participant time to symptom onset measures across replications when using the CTCAT.Evidence of convergent validity was obtained by comparing the CTCAT time measures to 10-point Likert scale pain measures for sessions 1 and 2. That is, high levels of pain felt by the participant during the test was expected to produce a short time to symptom onset. After conducting a Pearson’s moment correlation, the results revealed a strong significant negative correlation between time and pain, r= -.925, p< 0.001, n= 10 for session 1 and a moderate marginally significant correlation between time and pain, r= -.574, p= .082, n= 1 for session 20. Moderate correlation for session two may be related to participants’ variability on symptom onset based on their activities of daily living conducted during the day. This outcome, however, provides some convergent-related evidence of validity for the use of the CTCAT time measures to assess CTS.Evidence of discriminate validity was provided by correlating the CTCAT time measures during the CCT to the BCTQ pain measures before the CCT, which were not expected to correlate. Pearson’s moment correlation revealed no significant correlation between the BCTQ pain measures before the CCT and CTCAT time measures during the CCT for session 1, r= .128, p= .724, n= 10 and session 2, r= .017, p= .963, n= 10.
4. Discussion
- The results of the current study are discussed in terms of prototype development; theoretical and empirical rationales to assess pain. The measurements of pain are discussed and used as criterion to provide evidence of reliability and validity for the use of the CTCAT time measures as a technique to assess CTS.
4.1. Prototype Development
- Developing a new assessment tool requires time, patience, and ingenuity. It also requires evidence of reliability and validity for the use of the tool measures [15]. In the current study, a CTCAT device was created by the researchers based on theory and clinical concepts to assess CTS [20]. The initial tool prototype developed was too simple, and it did not allow the user to control the pressure steadily for the required time of testing. This design was abandoned and a new prototype emerged.The new CTCAT prototype was instrumented with a restraint strap, an inflation pad, pressure gauge, quick-released strap mechanism, and pressure ball as shown in Figure 1. This modification, in combination with the addition of the restraint strap, allowed pressure to be applied evenly and steadily for the duration of the testing and be released quickly at the completion of the test by activating the release strap mechanism.After the researchers were satisfied with the new CTCAT prototype, the study began by first examining the reliability and validity of BCTQ and 10-point Likert scale as measures of pain to assess CTS. These measures of pain were later used as criterion to validate the CTCAT measures of time as a new tool to assess CTS.
4.2. Boston Carpal Tunnel Questionnaire
- According to Sambandam et al. [26], the BCTQ developed by Levine et al. [19] is the most commonly used outcome measure of pain in the assessment of patients with CTS. The inter-item and intraclass reliability analyses in the current study provided consistent results for part one and two of the BCTQ. These outcomes support the literature [3, 26] and indicate that the BCTQ measures produce reliable results to be used as validity criterion for further analysis. Similarly, strong correlations were found across replications of the BCTQ measures. Since the scores of this questionnaire were shown to be consistently reliable not only within testing sessions, but also between testing sessions, there is a strong evidence to suggest that the inferences made from the test score interpretations of the BCTQ consistently measure pain and difficulty with certain daily functional activities as found in previous studies [26].
4.3. Ten-point Self-report Likert Pain Scale
- Since the BCTQ does not provide measures of pain during the administration of CCT, but rather provides measures of pain symptoms and difficulty with activities of daily living within 24 hours before the administration of the CCT, it was crucial to develop a 10-point Likert scale to measure the amount of pain felt by the participant during the administration of the CCT. To assess the reliability of the 10-point Likert pain scale, intraclass correlation analyses were conducted that compared the pre-test pain scores for testing session one to the pre-test pain scores for testing session two. Strong to moderate significant intraclass correlation coefficients revealed consistency across replications of the 10-point Likert scale. Over the two testing sessions, the Likert pain scale scores for all participants groups were found to be significantly reliable. This outcome supports the reliability literature, which is solely concerned with how test scores are expected to vary when repeatedly using an assessment tool [13].To provide evidence for the validation of the 10-point Likert pain scale, the concept of validity by Messick [35] was used, which states that “validity is an integrated evaluative judgment of the degree to which empirical evidence and theoretical rationales support the adequacy and appropriateness of inferences and actions based on test scores or other modes of assessment…it is important to note that validity is a matter of degree, not all or none” (p.13). Messick addressed the word integrated, which, in his concept of validity, referred to the summation of many sources of information based on the existing evidences and the interpretation of test scores. In the current study, empirical evidence and theoretical rationales in combination with different sources of information based on quantitative and qualitative information were used to validate the 10-point Likert pain scale measures including concurrent-related, construct-related, and face-related evidence of validity.According to Standards for Educational and Psychological Testing [30], concurrent-related evidence of validity is “the degree to which the criterion information obtained from a sample of items, tasks, or questions on a test relate to the same criterion information obtained from a standard when both measures are obtained simultaneously” (p. 10). For this study, concurrent-related evidence of validity was provided by comparing the 10-point Likert pain scale pre-test pain measures to the BCTQ part one (symptom severity) measures when the two assessments were administered simultaneously. The similarity between the two instrument measures was assessed by first putting the two instrument pain measures under the same scale and conducting intraclass correlational analyses for both testing sessions. Strong significant correlations between the two instrument pain measures provided construct-related evidence of validity for the use of the 10-point Likert scale as an instrument to measure pain related to CTS.Comparing the pre-test measures of pain of the 10-point Likert scale and not the post-test measures was necessary because the BCTQ only measured pain discomfort for the last 24 hours and not during the administration of the CCT. Furthermore, comparing the BCTQ pain scores to the 10 point Likert scale post-test measures would not provide concurrent-related evidence of validity as CTS participant pain levels were more likely be increased due to the administration of the compression test causing differences in participant pain tolerance level. Significant correlation using the pre-test measures, however, provided evidence for the use of the 10-point Likert pain scale as validity criterion to assess CTS pain for later analyses.Evidence of face validity was also provided for the use of the 10-point Likert scale under theoretical and empirical rationales stated by Kane [15] and Messick [35]. Evidence of face validity was provided by using qualitative and quantitative measures to assess the degree to which the 10-point Likert scale measured participant pain during the administration of the CCT. These outcomes were also supported by qualitative data, as two participants reported that they had CTS symptoms brought on after the testing session was completed. More specifically, one participant reported that the symptoms lasted one hour after the physical testing, while the other participant reported a five minute flare up after the test.
4.4. The CTCAT as a CTS Assessment Tool
- While self-report questionnaires, such as the 10-point Likert scale and the BCTQ, have the advantage that they can be easily implemented to capture participant information (e.g., pain measures) [14], the responses may contain a subjective source of bias that can affect the variance of the intended construct being measured [36]. One avenue to minimize the degree of subjectivity as a source of noise embedded within participant responses when using self-report measures (e.g., pain measures) is to include direct measures [23, 37]. Including direct measures in the assessment in conjunction with subjective measures creates a test battery of measures that minimize threats to the validity of the data [23].In the current study, a CTCAT was designed to minimize the subjectivity imparted by a medical professional when administering the CCT. The CTCAT was also designed to provide another source of evidence in CTS diagnoses when administering the CCT [7]. By using response time measures during the administration of the CCT in conjunction with self-report measures of pain, it may be possible to more accurately assess individuals with CTS. This approach was implemented based on the concept of validity, described by Kane [15] and Messick [35] and the research work of Love [20].The CTCAT designed as an assessment tool for CTS in the current study has convergent and discriminant evidence of validity. Convergent validity according to Campbell and Fiske, is the degree to which two constructs that are believed to be related, are in fact, related. In the current study, convergent-related validity was provided by correlating the response time measures to the amount of pain felt by the participant during the administration of the CCT. Higher negative correlations for non-CTS and CTS participants were found for testing session 1 and moderate correlations for testing session 2. Moderate significant correlation scores for testing session two, however, may be due to the participants’ variability in daily pain symptoms as indicated by Love [20]. Furthermore, negative correlations between response time measures and self-report measures during the administration of the CCT indicated that as the participant’s amount of pain felt during the administration of the CCT increased, the response time to CTS symptom onset decreased [20, 38].Discriminant-related evidence of validity is the degree to which measures that are believed to be unrelated are, in fact, truly unrelated [15, 38]. The current study hypothesized that the response time to symptom onset during the administration of the CCT would not correlate to measures of pain before the administration of the CCT. This hypothesis was formulated based on Love’s [20] theoretical rationale, that in order to trigger a participant’s CTS symptoms, compression of the median nerve must take place over time.Non-significant correlation results with the pain measures before the administration of the test indicated that the CTCAT response time measures depended only on triggering participant’s CTS symptoms by compressing the median nerve over time. This outcome supports Love’s rationale and this study’s hypothesis. It also provided discriminant-related evidence of validity as defined in the literature [15, 38] for the use of the CTCAT response time as a valid measure to access CTS when combined with self-report measures of pain.The outcome of this study also revealed that using the CTCAT to elicit participant CTS symptoms and pain supports the research of Durkan [7] and the findings put forth by Love [20]. As found through the current research, in order to cause a change in participants’ pain and symptoms, the median nerve had to be compressed for a period of time; in this case, no longer than 30 seconds. The use of the CTCAT also builds on the research of Durkan [7] as the testing revealed that a steady, direct pressure roughly the size of the thumb pads held over the median nerve is required to reproduce a participant’s CTS pain and symptoms. When used on CTS participants who have not had decompression surgery, the CTCAT elicited symptoms in all participants on both testing occasions.The potential use of the CTCAT in the clinical setting does not completely support Reuben and Siu [23], who stated that objective assessment measures may require more staff time and effort, resulting in higher operating costs. As the CTCAT physical testing can be carried out by one person, and is non-invasive, it could, therefore, reduce diagnostic and assessment wait times and costs associated with diagnosis and assessment of CTS.The reliability for the CTCAT response time measures was assessed by comparing the administration of the CCT for testing sessions one and two using an intraclass correlation. Highly significant reliability results in terms of intraclass correlation time scores suggest that over the two testing sessions, the amount of time that passed before the participants needed to end the physical testing was roughly the same. This outcome provided evidence of reliability for the use of the CTCAT response time measures across replications of the CCT. These results also support Love’s [20] statement that in order to trigger participant CTS symptoms, compression of the median nerve must take place over time.
5. Conclusions
- This study examined the potential usefulness of the CTCAT for clinical settings to assess CTS when compared to the BCTQ and the 10-point Likert pain scale. The study was conducted because it is evident that a combination of subjective questionnaires and simply administered objective testing measures better diagnose CTS in the general population. As stated in the literature, the irritation of the median nerve in the carpal tunnel space produces CTS symptoms such as tingling, burning, general discomfort, and aching in the affected fingers [2], which are problematic symptoms for individuals to perform his/her activities of daily living and work tasks.It was found that the CTCAT could then potentially be used to assess and diagnose CTS within the clinical setting due to the success rate observed when assessing those with CTS who had not had surgical release and decompression. From the practical perspective, this outcome provides an avenue for health professionals and patients to assess CTS symptoms with a simple technique composed of a 10-point Likert scale pain questionnaire and a CTCAT with moderate to strong evidence of reliability and validity measures.As the CTCAT gives reliable and valid results without being technologically complex, medical professionals could easily use it to assess and diagnose CTS in a clinic setting. Introducing a simple to use assessment tool to the healthcare system would greatly reduce the wait periods associated with more complex objective testing measures such as NCS and EMG, improving the speed with which accurate CTS diagnoses can be made.
5.1. Limitations
- The primary limitation on this study was the small sample size of participants with CTS. Initially, this study aimed to include 40-50 participants with CTS to the testing group. Although a fairly large number of medical clinics were approached within the region, some professionals were either not interested in helping to recruit participants, or were not currently treating any patients in their clinics with CTS. The study, however, aimed at the development of an objective measuring instrument for assessing CTS to potentially be used in the clinical setting.
5.2. Future Research
- Future research in this area should include a larger sample size of participants with CTS to strengthen the evidence of the reliability and validity of the CTCAT response time measures. Furthermore, the research should also include other clinical exams and diagnostic imaging such as functional magnetic resonance, electromyography, or nerve conduction studies to examine the accuracy of the CTCAT response time measures to better gauge the sensitivity and specificity of the device when assessing CTS in the clinical setting.An additional suggestion for future research will be to assess CTS individuals based on his/her chosen career path. If research participants were divided into career classes, such as desk jobs or manual labour jobs, the CTCAT testing may be able to also assess whether or not one type of job versus another produces more severe cases of CTS. This approach may be beneficial for employers and medical professionals to help provide appropriate ergonomics advice and assist with clinical decisions regarding treatment. Lastly, future research could look at adapting the CTCAT to be a more compact portable tool.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML