-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Practice
p-ISSN: 2326-1463 e-ISSN: 2326-1471
2016; 5(1): 1-5
doi:10.5923/j.cp.20160501.01

Comparison of Surgical Techniques in T1 and T2 Thymomas: The Possibility of VATS Thymectomy Compared with Open Resection
Madiyorov B. T. 1, Krotov N. F. 2, Rasulov A. E. 1, Chernyshova T. V. 1, Sabirov D. R. 1, 3
1National Cancer Research Center, Ministry of Healthcare of the Republic of Uzbekistan, Uzbekistan
2FGBI "Oncology Institute named by N.N. Petrov" of Russian Ministry of Health, Uzbekistan
3Head of the Military Medical Faculty at the Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Madiyorov B. T. , National Cancer Research Center, Ministry of Healthcare of the Republic of Uzbekistan, Uzbekistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

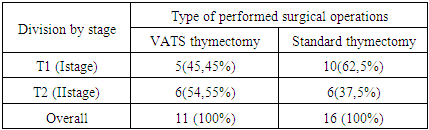
Background: The objective of this study was to compare the results of VATS thymectomy, with standard operations (thoracotomy, sternotomy), and assessment of overall survival in the long term. Patients and Methods: A retrospective review of 27 patients who underwent thymectomy, in last 13 years. The key point of the study was overall survival in the two groups. Results: Of the 27 patients, 11 patients have undergone VATS thymectomy in stage I and II; in 16 patients thymectomy with standard access in stages I and II was performed. Postoperative mortality was 0%. Stage of the tumor and the number of patients undergoing radio and chemotherapy, were comparable in both surgical groups. The average length of stay in the hospital for the main group was 7 days, in the control group 16 days. Essential differences in index of 5-10 year survival have not been identified between the 2 groups. Conclusions: VATS thymectomy is an effective and less traumatic treatment for thymomas. There were no significant differences in patient survival depending on the used technique.
Keywords: Mediastinum, Videothoracoscopy, Thymoma, Thymectomy
Cite this paper: Madiyorov B. T. , Krotov N. F. , Rasulov A. E. , Chernyshova T. V. , Sabirov D. R. , Comparison of Surgical Techniques in T1 and T2 Thymomas: The Possibility of VATS Thymectomy Compared with Open Resection, Clinical Practice, Vol. 5 No. 1, 2016, pp. 1-5. doi: 10.5923/j.cp.20160501.01.
1. Background
- Thymus tumors according to different data make up from 10% to 60% of all tumors of the mediastinum, which constitute about 3-7% of all oncologic pathology and 10- 20% of primary mediastinal disorders. [1-10, 11, 13-15, 20, 22]All variety of thymic tumors are divided into 2 groups: I group consists of tumor histogenesis which is associated with the epithelial component of the thymus gland (thymoma, thymic cancer); Group II - tumor histogenesis which is not associated with the epithelial component (carcinoid, germ cell tumors, lymphomas, soft tissue tumors, hyperplasia and thymic cysts) [3, 7, 16, 19, 22].To date, histological classification of thymomas proposed by WHO experts, under the general editorship of J. Rosai was accepted in 1999 and modified in 2004: spindle cell (type A), lymphoid (type B1), mixed (type AB, B2), epithelial (type B3), a cancer of the thymus (type C). [11, 16, 19].For staging, prognosis, and surgical treatment, thymoma classification based on the degree of infestation, the roposed Masaoka is used [5, 9]. 1st degree for this classification corresponds to the absence of macro- and microscopic invasion; 2nd degree: macroscopic invasion of the thymus tissue of the capsule; IIIa degree: no invasion of large vessels; IIIb degree: an infestation of large vessels; IVa degree: dissemination on the pericardium or pleura; IVb degree: Lymphogenous or hematogenous metastasis [2, 3, 9, 11, 13, 21].Until now, a surgical method is the "golden standard" in treatment of patients with tumors of the thymus gland. This is true both for the surgery and for the combined treatment. Radical surgery - the most important factor in prognosis of surgical treatment of patients with benign and malignant thymoma [2, 4, 15, 17, 19, 25, 26].Tumors of the thymus gland (thymoma), tumor-like growths, and gravis myastheniaare considered as the indications for surgical treatment. Approximately 30% of patients with thymoma manifests myasthenia gravis (MG) [1, 2, 6, 7, 12-15, 20]. Blalock, et al [1] is one of the first to describe a series of patients with myasthenia gravis (MG) for whom thymectomy was performed.The main factor determining the clinical manifestations, course and prognosis of thymoma, is the presence and extent of invasive growth. So according to various authors, surgical treatment is the key to long-term survival of patients, 5 and 10-year survival of patients with 1 and 2-stage thymoma after thymectomy, ranges from 89% to 100% and 71 to 95%, respectively, in stage III - 74% and 46% and the 5 year survival in disease IV is less than 25% [2, 3, 7, 13, 14].To perform radical surgery on the thymus using different approaches - neck; traspleural–right sided and transbipleural; and trasnssternal - by a total or partial median sternotomy and by oblique partial sternotomy [2, 4, 5, 7, 8, 12, 14, 17, 18, 20, 21, 26].However, with the recent widespread introduction of thoracoscopic techniques into clinical practice, is increasingly discussed the use of video assisted thoracoscopy (VATS) thymectomy that reduce surgical trauma and associated postoperative complications [4, 7, 8, 12, 15, 21, 23, 26].Many publications have demonstrated the safety and efficacy of VATS thymectomy in patients with myasthenia gravis. Many researchers are of the opinion that this surgery is currently the method of choice in these patients [4, 7, 8, 12, 15, 21, 23, 26].In general, it should be recognized that the VATS interventions research data in isolated tumors of the thymus are not accompanied by myasthenia, i.e. it is underrepresented, and the question about the possibilities of VATS access in such clinical situations requires further study.Given the above, the aim of this study was to compare the results of VATS thymectomy, versus standard operations (thoracotomy, sternotomy), and assessment of overall survival on the long term.
2. Subjects and Methods
- Retrospective analysis was held on 27 patients with tumors of the thymus, the first and second stages, in period from 2001 to 2014 who were treated in the thoracic surgery department. Patient characteristics: -13 males (48.15%) -14 female (51.85%), age varied: from 10 to 55 years. The mean age was 31,40 ± 16,4 years. To evaluate the general condition of the patient, verification of tumor process carried out comprehensive investigations: general clinical research biplane standard chest X-ray, bronchoscopy, trans-thoracic puncture of the tumor (indication), and cytological and histological examination of the tumor.For differential diagnosis used additional methods of investigation: CT scan of the chest with contrast mediastinal vessels, ultrasound examination of the mediastinum, supraclavicular area, abdomen, magnetic resonance imaging (MRI) of the chest. A number of patients used the following diagnostic procedures: supraclavicular lymph node puncture or prescalenous biopsy, thoracoscopic study.The main source of information about the further development of the disease was hospital records, which contain information on the results of periodic inspections, information from the database of organizational and methodical department and response from the regional oncologic dispensary of the republic, which was received according to request.Depending on the approach of surgery, patients were divided into two groups: basic - VATS thymectomy, the control - thymectomy performed through the standard approach - thoracotomy, sternotomy. Comparison between parameters of both groups by stages is summarized in Table 1.
|
3. Results and Discussion
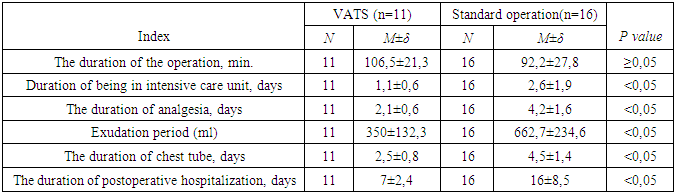
- In all VATS, patients did not have dangerous complications. Conversion was performed in 1 (9.1%) patients in the early stages of the development of techniques in our clinic. The reason for the conversion in this case was the presence of pronounced adhesions in the pleural cavity.Traumatism of surgical interventions was evaluated on the basis of exudation in the postoperative period, the timing of draining, time spent in the intensive care unit, duration of surgery, and duration of anesthesia (Table 2). In the long-term period: time to recurrence and survival after surgery, depending on the surgical approach.
|
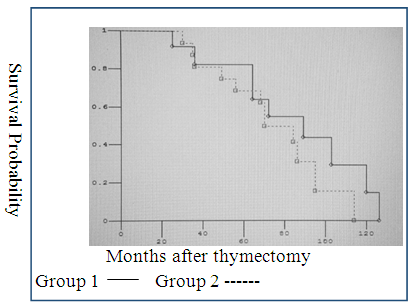 | Figure 1. The survival rate after VATS thymectomy at T1 and T2: Group 1 - thymoma T1; Group 2 - thymoma in T2 |
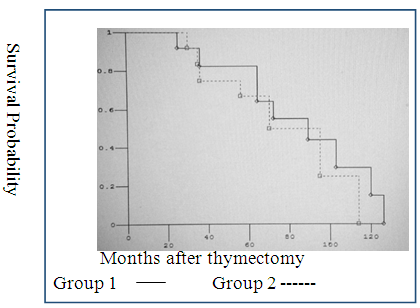 | Figure 2. The survival rate after standard thymectomy at T1 and T2: Group 1 - thymoma T1; Group 2 - thymoma in T2 |
4. Conclusions
- Thus, method of video assisted thoracoscopic surgery in thymomas decreases amount of exudation, reduces drainage time after surgery versus thoracotomy and sternotomy VATS is an effective and less traumatic treatment for thymomas. Significant differences in patient survival depending on the used technique were not established.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML