-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Practice
p-ISSN: 2326-1463 e-ISSN: 2326-1471
2014; 3(1): 4-6
doi:10.5923/j.cp.20140301.02
Burned-out Testes Seminoma without Distant Metastasis
Hui-Ming Chung1, Ming-Dar Woon2, Fu-Jinn Lo3
1Department of Urology, Mennonite Christian Hospital, Hualien, 970, Taiwan
2Department of Oncology, Mennonite Christian Hospital, Hualien, 970, Taiwan
3Department of Pathology, Mennonite Christian Hospital, Hualien, 970, Taiwan
Correspondence to: Hui-Ming Chung, Department of Urology, Mennonite Christian Hospital, Hualien, 970, Taiwan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
While complete regression of a primary testicular tumor with progressive growth of metastases is well recognized in cases of non-seminomatous germ cell tumors, this phenomenon is rarely reported with seminoma. We report a case of a spontaneously regressed testicular seminoma without accompanying metastatic lesions. A 33-year-old man had suffered from persistent scrotal induration and transient testicular pain of the left testicle for one month when he visited our hospital. He had undergone left orchiopexy for undescended testes in his early childhood. Serum levels of Alpha-fetoprotein, beta-human chorionic gonadotropin, and lactate dehydrogenase were all within normal limits. After 4 weeks of antibiotics treatment with cephalexin monohydrate, the left testicular mass remained unchanged. The patient was subjected to left radical orchiectomy. Histological examination revealed a fibrotic tumor with a small area of seminoma undergoing spontaneous regression. Postoperative imaging showed no concomitant metastatic lesions. Adjuvant radiation therapy was provided. The patient was free of disease at 2 years follow-up after surgery. We recommend that long term follow-up of patients with undescended testes, even if atrophic, is necessary for early detection of possible testicular tumors.
Keywords: Burned-out, Metastasis, Seminoma, Spontaneous regression, Testes
Cite this paper: Hui-Ming Chung, Ming-Dar Woon, Fu-Jinn Lo, Burned-out Testes Seminoma without Distant Metastasis, Clinical Practice, Vol. 3 No. 1, 2014, pp. 4-6. doi: 10.5923/j.cp.20140301.02.
1. Introduction
- “Burned-out” tumor of the testes refers to spontaneous regression of testicular tumors. It was typically diagnosed at the metastatic stage when traces of tumor were found in the testes[1]. Most of the reported cases with spontaneous regression of testicular tumors have occurred in patients with non-seminomatous germ cell tumors. Reports of spontaneously regressed primary testicular tumors with seminoma are very rare[2, 3]. We report a case of burned-out testicular seminoma without accompanying metastatic lesions.
2. Case Report
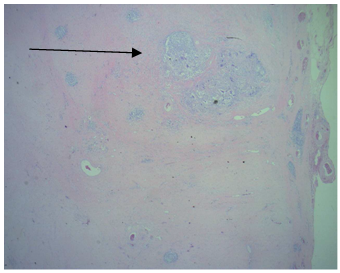
- A 33-year-old man presented with a 1-month history of intermittent left testicular pain with induration. No other subjective discomfort was reported. He had a history of orchiopexy for his left undescended testicle since the age of 2 years. Medical and family histories were otherwise unremarkable. The patient had a normal psychosexual status. He was married, and he had a 2-year-old son. Physical examination showed a generally normal young man, and the left testicle was atrophic, firm, and somewhat tender. Serum levels of the tumor markers for germ cell tumors, including alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (beta-hCG), and lactate dehydrogenase (LDH), were within normal limits. The scrotal ultrasonography was not contributory other than identifying an atrophic testicle with nonspecific internal calcifications in the left-side hemiscrotum.The scrotal induration persisted after 4 weeks of oral antibiotics (cephalexin monohydrate) under the impression of epididymitis. He then was subjected to left radical orchiectomy for the diagnosis of a testicular tumor. A solid, 5 cm testicular mass with no evidence of local extension was removed. Macroscopically, the left testicle was replaced almost completely by a well-demarcated, white, fibrotic nodular scar measuring 4.3x3.2x2.9 cm (figure 1). The tunica albuginea, epididymis and vas deferens were normal. The histopathological examination showed a small area of seminoma within the fibrotic tumor. Testicular atrophy was demonstrated by shrunken tubules with decreased or absent spermatogenesis and thickened peritubular basement membrane peripheral to the scar (figure 2). Testicular scarring and atrophy are evidence of germ cell tumor regression in burned-out seminoma. The seminomatous lesions were also positively immunostained using CD117 monoclonal antibodies, which can differentiate seminoma and nonseminoma (figure 3). Concurrent image study including chest, abdomen, and pelvic CT did not reveal any metastatic lesions. With a stage 1 disease and with a relatively excellent prognosis, an active surveillance was recommended. But the patient declined the offer and asked for a more aggressive therapy to reduce the risk of recurrence. After a thorough discussion with the patient regarding the two adjuvant treatment options, including chemotherapy and radiation therapy, he refused chemotherapy based on personal reasons. Then he was put on the adjuvant radiation therapy consisting of 30 Gy to the paraaortic lymph nodes without adverse effects. The patient was free of the disease at 2 years after the orchiectomy without any evidence of recurrence or metastatic lesions on the follow-up CT scan.
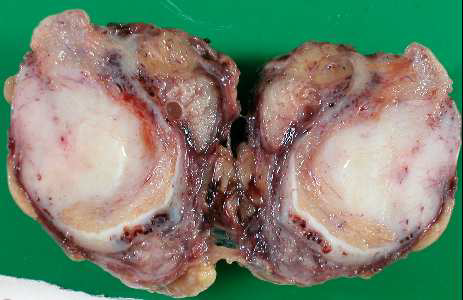 | Figure 1. Gross appearance of the atrophic testes with a well-demarcated nodular scar |
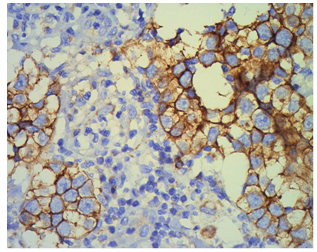 | Figure 3. The seminomatous lesions were positively immunostained using CD117 monoclonal antibodies, which can differentiate seminoma and nonseminoma |
3. Discussion
- The burned-out phenomenon of germ cell tumors has been described by several authors, but most cases were nonseminomatous germ cell tumors accompanied by metastatic lesions[2, 4]. Spontaneously regressed testicular tumor with no accompanying metastatic lesions has rarely been reported. This patient is an exceptionally rare case with a burned-out testicular seminoma without concomitant metastatic lesions. An enlarged testicular mass, especially if painless, usually raises the concern of a possible testicular malignancy. However, an undescended testis after orchiopexy may become atrophic and firm in adulthood. In such cases, detection of a regressed testicular tumor could be difficult, leading to neglect of the tumor in the absence of extragonadal metastatic lesions. A high index of suspicion and careful scrotal examination is therefore always required in order to diagnose the possibly regressed testicular tumor. While palpation of the testes may not be a sensitive method, some authors suggest that high-resolution ultrasonography can identify a small tumor with echogenic foci that represent the remnants of a regressed tumor. It was reported that burned-out testicular tumors may sometimes occur with calcifications[1]. Histological features that can help in establishing a diagnosis of burned-out testicular tumors include scar formation, testicular atrophy, intratubular calcification, lymphoplasmacytic infiltrate, and hemosiderin - containing macrophages[5]. The mechanisms of the burned-out phenomenon for testicular tumors remain unclear, though some immunological and ischemic mechanisms have been proposed[6]. An immunological hypothesis suggests that after repeated exposure, common tumor antigens can be recognized by cytotoxic T lymphocytes and subsequently replaced by fibrosis[7]. For an early-stage seminoma such as in this case, the prognosis is excellent after the radical orchiectomy. More than 80 percent of patients with stage 1 seminoma are cured with radical orchiectomy. Active surveillance is therefore recommended after surgery if the patient is willing and able to comply with follow-up[5]. For those who desire a more aggressive treatment to further reduce their risk of recurrence, adjuvant treatment options include chemotherapy (with single agent carboplatin) and radiation therapy (to the paraaortic lymph nodes). Both options provide similar 5-year relapse-free rates (over 90 percent)[8, 9]. The former is preferred—especially for those who wish to preserve fertility—because it is associated with less long term toxicity. Single agent carboplatin, rather than a traditional cisplatin-based combination, has been used because it is associated with less toxicity[8]. However, it still carries some risk of acute side effects, including thrombocytopenia, nausea, and vomiting. Potential acute side effects of adjuvant radiation therapy include fatigue, nausea, vomiting, myelosuppression (typically mild), tanning of the skin under treatment, and impaired fertility. Adjuvant radiation therapy may also be associated with an increased risk of secondary malignancies and a possible increased risk of cardiovascular disease. The traditional radiation therapy involved a broader field, including paraaortic lymph nodes and pelvic lymph nodes. The paraaortic lymph nodes are the initial nodal group to be involved in the majority of patients who experience tumor spread. The newer technique with radiation to the paraaortic lymph nodes alone reduces the size of the treatment field and the dose delivered. This approach yields similar overall recurrence and survival rates compared to the traditional approach, but significantly decreases the treatment-related morbidity[10].Three tumor markers, including AFP, beta-hCG, and LDH, have established role in the management of germ cell tumors. Serum levels of AFP and/or beta-hCG are elevated in 80 to 85 percent of men with nonseminomatous germ cell tumors. In contrast, serum beta-hCG is elevated in fewer than 25 percent of seminomas, and AFP is not elevated in pure seminomas. Therefore, serum tumor markers have very low utility in the follow-up of men with stage 1 seminoma[11]. The serum levels of the three tumor markers were within normal limits in our case. It is not necessary to follow them as part of the surveillance protocol. We conclude that careful regular follow-up of patients with undescended testes, with or without orchiopexy, is necessary for early detection of possible testicular tumors. In the presence of an atrophic testicle with induration, whether or not with metastatic lesions, a high index of suspicion of burned-out testicular tumors (although relatively rare) must always be maintained.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML