-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Control Science and Engineering
p-ISSN: 2168-4952 e-ISSN: 2168-4960
2020; 10(1): 16-21
doi:10.5923/j.control.20201001.03
Received: Sep. 9, 2020; Accepted: Oct. 2, 2020; Published: Oct. 15, 2020

Effects and Control of Chemical Composition of Clinker for Cement Production
Sanusi Nuhu1, 2, Samaila Ladan3, Abubakar Umar Muhammad4
1Department of Chemical Engineering, Umaru Ali Shinkafi Polytechnics Sokoto, Nigeria
2MSc Student, Department of Chemical Engineering, Ahmadu Bello University Zaria, Nigeria
3Monitoring and Evaluation Department, Tertiary Education Trust Fund, Nigeria
4Faculty of Pure and Applied Science, Usman Danfodiyo University Sokoto, Nigeria
Correspondence to: Sanusi Nuhu, Department of Chemical Engineering, Umaru Ali Shinkafi Polytechnics Sokoto, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This research focused the role to study the effects and ways to control the chemical composition of clinker for better cement production. Cement is a substance produced by grinding a mixture of a clay and limestone and heating to a temperature of 1450°C, in which the chemical transformation occurs inside the kiln to form new compound called clinker. The methodology of this research work are; the 10 samples of clinker were collected on the apron conveyor, 10g of each sample were weighted, milled and pelletized with the aid of pyridine and binding agent. Then the sample were subjected to XRD analyzer machine for determination of mineralogical composition of clinker, the minerals and oxides detected are; C3S, C2S, C3A and C4AF and CaO, SiO2, Al2O3 and Fe2O3. The other cement modulus such as LSF, AM, and SM were calculated using Bogues equation. From results fig.8 shows increases of early strength of cement as a result of increases of AM, fig.10 shows the decrease of early strength of cement as a result of the increase of SM, fig 9 also is the chart of LSF and free lime (FCaO) and the result shows how the free lime increase as the LSF increases, which leads to requires more energy consumption for clinker formation, poor quality clinker, volume expansion and poor strength of cement. Therefore the chemical composition of cement raw materials and clinker are critical to cement plant efficiency and energy consumption. To ensure constant and consistent chemical compositions and quality of cement clinker with lowest possible energy consumption, attention must be paid to kiln feed and clinker chemical compositions.
Keywords: Chemical, Cement, Clinker, Composition, Kiln, Consumption
Cite this paper: Sanusi Nuhu, Samaila Ladan, Abubakar Umar Muhammad, Effects and Control of Chemical Composition of Clinker for Cement Production, International Journal of Control Science and Engineering, Vol. 10 No. 1, 2020, pp. 16-21. doi: 10.5923/j.control.20201001.03.
Article Outline
1. Introduction
- Effects and control of chemical composition of clinker is an important of the cement manufacturing process. In cement production the effects of chemical composition of clinker in the process materials and finished products has to be analyzed, monitored and effectively control to optimize the process and a consistent desired product quality of cement.Cement substance is produced by grinding a mixture of a clay and limestone together and heating to a temperature of 1450°C. In which the chemical transformation occurs inside the kiln to form new compound called clinker. Clinker formed from burning of kiln stage, it can be described as lumps or nodules usually 3 mm to 25 mm in diameter. CaO, SiO2, Al2O3 and Fe2O3 are the major component of cement clinker, they accounting for more than 95% and MgO, TiO2, P2O5 and alkalis are the minor components in initial less than 3% they are not present in individual oxide, but exist as compound formed by two or more oxides (Mohammd A. Aldies, et al 2010). C3S, C2S, C3A and C4AF are the composition minerals of the clinker and these minerals is as a result of the pyro processing or reaction of oxides in the kiln which leads to the formation of lime saturation factor (LSF). LSF is the ratio of CaO to SiO2, Al2O3, and Fe2O3. Clinker with LSF close to or beyond 1.0 indicate a likelihood of the presence of free lime, this will results to the high burn of clinker and hence difficult in grinding (Nuhu.S, et al 2019). However LSF also control the proportion of C3S to C2S in clinker, high value of LSF causes free lime (CaO) not combine with these oxides, stay as free lime and excess free lime leads to undesirable effects such as increases setting time, difficult in grinding of clinker, volume expansion and reduces strength of cement (CCNN-Sokoto, 2013). Therefore from the chart figure 9 below confirms that chemical composition of cement clinker are critical to energy consumption, because the grinding of cement clinker depends on LSF and free lime compositions which any slide deviation from these composition can significantly affect the performance of the installation, that’s including the energy consumption and quality of the cement. This research paper focused to study the role of the effects and ways to control the chemical composition of clinker for better production of quality cement with lowest possible energy consumption. The study was done based on the analysis of the clinker chemical compositions obtained from Sokoto cement plant.
1.1. Raw Feed Chemistry
- In order to achieved the required clinker chemical composition during sintering the raw feed chemistry must be set. Different chemical parameters can be used for controlled depends on the number of raw materials used to prepare the feed. Usually the number of target parameters which can be controlled is one less than the number of raw materials. However also raw mix control using calcium carbonate requires two raw materials – high grade and low grade stone, if the iron content must also be controlled then a third raw material containing iron oxide must be added in order proper adjustment (CCNN - Sokoto, 2009). Normally the raw feed target applied at a plant is the available raw materials and the important clinker parameters. Usual combinations for raw feed target parameters may be based on:§ Chemical composition – CaCO3, SiO2 and Fe2O3§ Calculated mineralogy of C3S, C3A and C4AF§ Calculated ratios such as LSF, SM and AM§ Or a combination of parameters from all three groups.
1.2. Burning Process
- The modern technology system of cement manufacture, the retention time of the material inside the kiln is 30 to 40 minutes, of which the large part is in burning zone. The temperature of the material increases rapidly from 850°C to 1250°C to 1300°C, at which temperature of clinker melt is, formed (Mohamed A. Aldieb, 2010). The chemical and physical changes of the material take place in the burning zone simultaneously; it is importance with respect to the kinetics of clinkerization reactions and agglomeration process. The actual temperature of melt formation depends on the chemical composition of the feed.However, the kiln operator needs to keep the temperatures stable in each part of the kiln system, in order to convert the raw materials (kiln feed) in the kiln into clinker minerals, because with the deviation of temperature, kiln operation become unstable and clinker minerals are not properly formed making cement performance variable (CCNN - Sokoto, 2009). Moreover too long heating the feed in the kiln would make the minerals in the clinker grow larger and hence the clinker become less reactive and produces a poor quality of cement.Fig.1 Below described the process of clinker formation. The primary clinker phases conclude transformation and properties (Mohamed A. Aldieb, 2010):§ The most important constituent is alite (C3S), 50-70% in normal Portland cement clinkers. It is tricalcium silicate (Ca3SiO5) modified in composition and crystal structure by ionic substitutions. It produces most strength up to and including 28 days. Each 10% increase in C3S content increases the 28 day EN 196 mortar strength by about 5 MPa (Mohamed A. Aldieb, 2010).§ Belite (C2S) constitutes 15-30% of normal Portland cement clinkers. It is dicalcium silicate (Ca2SiO4), it reacts slowly and improves later strength (≥28 days).§ Aluminate (C3A) constitutes 5-10% of normal Portland cement clinker; it is tricalciumaluminate (Ca2Al2O6). It releases a lot of heat, shortens setting time and improves very early strength, but it lead the cement to make prone to sulphate attack.§ Ferrite (C4AF) it constitutes up to 5-15% of normal Portland cement clinkers. It is a tetracalcium aluminoferrite (Ca4AlFeO5). It make little effect on strength but it gives cement the dark coloring.
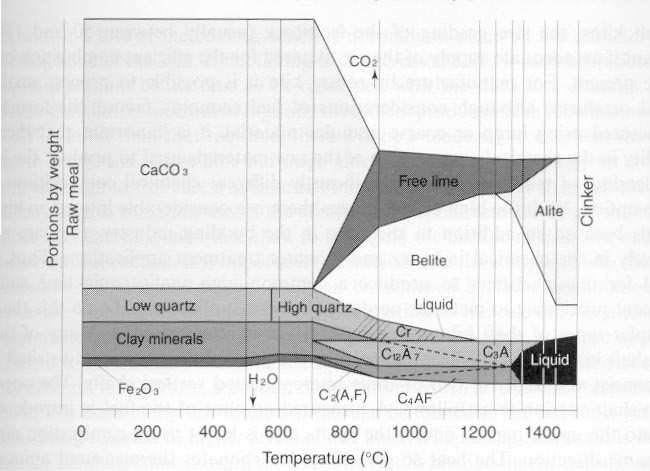 | Figure 1. A schematic views of the clinker formation reactions (markus, 2003) |
2. Materials and Methods
- The materials and methods used in carrying out chemical analysis using x-ray diffraction machine in cement industry to determine the presence and amount of minerals species in samples, as well identify phases. And method for compressive strength test.
2.1. Materials
- § Distilled water§ 10 Samples of weighted of clinker § Compressive strength testing machine§ Jolting machine§ The prism mould§ Mixer
2.2. Experimental Procedure
- § The 10g of each clinker samples were weighed, milled and pelletized with the aid of pyridine and binding agent.§ Pelletized samples were subjected to XRD/XRF analysis machine, this were done to determine the mineralogical chemical compositions of the clinker samples and their respective elemental oxides. The clinker minerals whose compositions were determined using XRDF/XRD were; C3S, C2S and C4AF, while the elemental oxides compositions determined also are: CaO, SiO2, Al2O3, and MgO. The silica modulus, alumina modulus, lime saturation factor were found from the elemental oxides compositions using Bogues equation stored as a program in the XRD/XRF analyzer.§ For the compressive strength. 1350g, 450g and 225g of standard sand, cement sample and distilled water were weighed respectively and then mixed in the automatic mixing machine. The mixtures then transferred into the prism mould mounted to the jolting machine and jolted for 2 minutes. The prism mould then remove away for curing 24 hours inside curing chamber and then de mould it to remove cube at different periods 2days, 7days and 28days. Then the cube were remove from curing chamber to the compressive strength testing machine, then cube were placed on the resting point on the machine and were operated at low speed and reading were taken. § The free lime was determined using wet chemistry method.§ Lime saturation factor (LSF)The lime saturation factor is a measure of the degree of conversion of silica, alumina, and iron oxide into the corresponding lime and it can be calculated using the formula:
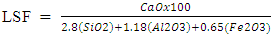 LSF is usually between 93 and 97% for Portland cement clinker to get a good balance cement properties and reasonable burnability.§ Silica modulusSilica modulus essentially governs the proportion of silica phases in clinker or is the indicator of burnability of the feed or clinker and it can be calculated using the formula:
LSF is usually between 93 and 97% for Portland cement clinker to get a good balance cement properties and reasonable burnability.§ Silica modulusSilica modulus essentially governs the proportion of silica phases in clinker or is the indicator of burnability of the feed or clinker and it can be calculated using the formula: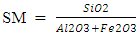 Silica modulus is one of the main components of C2S & C3S which make up the liquid phase and help in alite formation (Taylor and Francis Group, 2018).§ Alumina modulus The alumina modulus is also used as an indicator of burning temperature and flux characteristic in the kiln. It is the measure of the proportion of alumina to iron oxide in the mix, and it can be calculated using the formula:
Silica modulus is one of the main components of C2S & C3S which make up the liquid phase and help in alite formation (Taylor and Francis Group, 2018).§ Alumina modulus The alumina modulus is also used as an indicator of burning temperature and flux characteristic in the kiln. It is the measure of the proportion of alumina to iron oxide in the mix, and it can be calculated using the formula: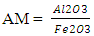 Alumina modulus of about 1.4 would leads to the formation of liquid phase or flux at lowest temperature in the kiln. Earlier formation of liquid phase or flux in the kiln allows alite formation to start sooner and go for a longer time, which improve the conversion of belite into alite. However AM with above or below these target 1.4, the amount of liquid phase would reduce at lower temperature, the length of burning zone coating would reduce, the flux becomes less fluid which slowing down alite formation, and subsequently more fuel is needed in order to obtain a given free lime clinker level (Taylor and Francis Group, 2018).
Alumina modulus of about 1.4 would leads to the formation of liquid phase or flux at lowest temperature in the kiln. Earlier formation of liquid phase or flux in the kiln allows alite formation to start sooner and go for a longer time, which improve the conversion of belite into alite. However AM with above or below these target 1.4, the amount of liquid phase would reduce at lower temperature, the length of burning zone coating would reduce, the flux becomes less fluid which slowing down alite formation, and subsequently more fuel is needed in order to obtain a given free lime clinker level (Taylor and Francis Group, 2018). | Figure 2. Concrete compressing machine |
 | Figure 3. Jolting machine |
 | Figure 4. Weigh balance |
 | Figure 5. Chemical analyzer |
 | Figure 6. Prism mould |
 | Figure 7. Curing chamber |
3. Results and Discussion
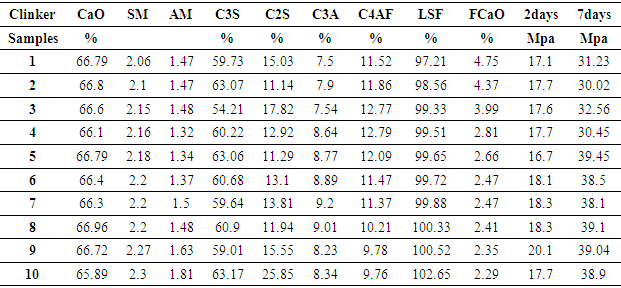
3.1. Table 1: XRD Analysis of Clinker Samples
|
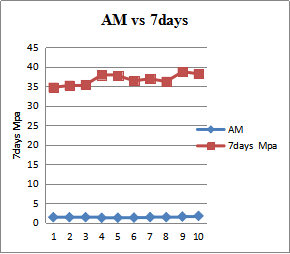 | Figure 8. Alumina Modulus and 7days Compresses Strength |
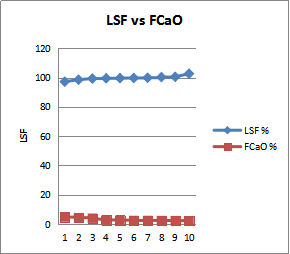 | Figure 9. Of Lime Saturation Factor and Free Lime |
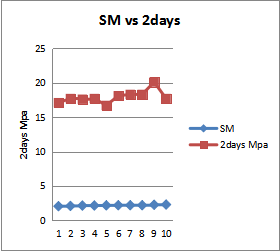 | Figure 10. Silica Modulus and 2days Compressed Strength |
4. Conclusions and Recommendations
4.1. Conclusions
- The quality of cement is typically assessed base on the compressive strength development mortar and concrete. The ingredient ratio of cement raw materials affects the quality and property of cement clinker and Portland cement, and also the optimal ingredient ratio promotes and stabilizes cement clinker. From the fig 8 shows increases of compressive strength as the AM increases, this is to confirm that, more formation of AM leads to the formation of liquid phase or flux in the kiln with lowest temperature, and early formation of liquid phase or flux in the kiln allows formation of alite which is the major component for early strength of cement. And fig 10 shows how the SM affects the early strength of cement. Point 3, 9 and 10 of SM draw down to point 20.1 and 20Mpa strength of 2days compressed strength, this is the fact that silica modulus is an indicator of the burnability of the feed to clinker. Increases of SM affect the formation of liquid phase, difficult in burning of clinker and poor quality of the cement, so attention must be paid to achieve the desired target of the SM. In fig.9 the results shows increase of the free lime because of the increase of LSF. However this to confirm that more LSF above the target leads to incomplete conversion of free lime, staying as free lime in clinker and hence these result to more energy require to burning the clinker, volume expansion, and poor quality of cement. Therefore the chemical composition of cement raw materials and clinker are critical to cement plant efficiency and energy consumption. However in order to ensure constant and consistent chemical compositions and quality of cement clinker with lowest possible energy consumption, attention must be paid to kiln feed and clinker chemical compositions.
4.2. Recommendations
- The attention should be paid during the pre –calcination of the kiln feed in the free calciner and correlation between the kiln feed and clinker LSF should also be done for elimination of the high free lime.
ACKNOWLEDGEMENTS
- Author acknowledge to department of chemical engineering Umaru Ali Shinkafi Polytechnic Sokoto, also for chief production Engineer, sokoto cement plant Engr Nasiru Bada for all support and encouragement.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML