-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2020; 10(2): 38-42
doi:10.5923/j.cmd.20201002.02
Received: Oct. 6, 2020; Accepted: Nov. 22, 2020; Published: Dec. 15, 2020

Interleukin-6 and Glial Fibrillary Acidic Protein in Prediction of Early Postoperative Cognitive Dysfunction after Orthopedic Surgery
Visolatkhon Sharipova, Azamat Alimov, Abror Valihanov
Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
Correspondence to: Azamat Alimov, Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Postoperative cognitive dysfunction (POCD) is associated with poor postoperative outcomes and these patients are at greater mortality risk compared to patients without neurocognitive decline. A prospective observational study involved 82 trauma patients undergone orthopedic surgery under general anesthesia. Perioperative cognitive assessment using neurocognitive tests and measurement of serum glial fibrillary acidic protein (GFAP) and interleukin-6 (IL-6) were performed. At the 7th day after surgery 13 (15.8%) patients had criteria for POCD diagnosis. Comparisons between patients with or without POCD at 7 days after surgery showed significantly increase in serum levels of GFAP and IL-6 in patients displaying POCD at the end of surgery and 24 hours after surgery respectively.
Keywords: Postoperative cognitive dysfunction, Orthopedic surgery, General anesthesia, Neurologic biomarkers, Glial fibrillary acidic protein, Neuroinflammation, Systemic inflammation, Neurocognitive tests
Cite this paper: Visolatkhon Sharipova, Azamat Alimov, Abror Valihanov, Interleukin-6 and Glial Fibrillary Acidic Protein in Prediction of Early Postoperative Cognitive Dysfunction after Orthopedic Surgery, Clinical Medicine and Diagnostics, Vol. 10 No. 2, 2020, pp. 38-42. doi: 10.5923/j.cmd.20201002.02.
Article Outline
1. Introduction
- Postoperative cognitive dysfunction (POCD), a condition that has been poorly recognized and defined for decades, is one of the most common postoperative complications, especially for the elderly [1].POCD is associated with poor postoperative outcomes. Patients classified as having POCD at the time of hospital discharge after noncardiac surgery are at greater risk of dying within 3 months than those without it, and if they have POCD at both hospital discharge and 3 months postoperatively, are nearly 5 times more likely to die 1 year later [2,3].It affects 25 to 41% of patients > 60 year of age at hospital discharge and 10-13% of patients 3 months after surgery [4]. POCD comprises a wide range of cognitive functions, including working and long-term memory, information processing, attention and cognitive flexibility, thus affecting quality of life and increasing mortality [5]. POCD pathogenesis has not been properly clarified, but advanced age, pre-existing cerebrovascular and systemic vascular disease and systemic inflammation have been considered important risk factors [6]. Neuroinflammation may cause neural cell dysfunction or death, leading to increased blood levels of biochemical markers for brain damage [7].
2. Methods
2.1. Study Population
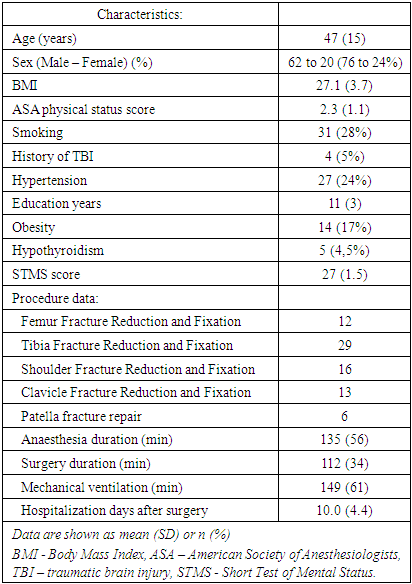
- 82 adult patients aged 30 and older who undergone trauma surgery under general anesthesia were considered to be eligible for this prospective observational study. Clinical, demographic and procedure data are summarized in Table 1.
|
2.2. Neurocognitive Tests and Criteria for Diagnosis of POCD
- The perioperative neurocognitive assessment protocol in 2 languages (Uzbek and Russian) was used to evaluate general cognitive function and identify patients with POCD. Patients were assessed before surgery and 7 days after surgery by applying a validated cognitive test battery: Auditory Verbal Learning Test (AVLT), Trail Making Test (TMT), Stroop Colour Word Test (SCWT), Digit Span Test (DST) and Digit-Letter Substitution Test (DLST). In summary, the tests are as follows: 1-AVLT. The test assesses immediate memory span, learning, susceptibility to interference and recognition memory. It consists of the same 15 nouns read aloud, with a 1 s interval between words, for three consecutive trials (AVLT-1, AVLT-2 and AVLT-3); each trial is followed by a free recall test. A delayed recall of these 15 words (AVLT-4) was performed 20 min after the third trial. Instructions were repeated before each trial to minimize forgetting. We evaluated the number of words recalled for each presentation, adding up the words recalled total result was registered [9]. 2-TMT. It evaluates the executive processes i.e. the frontal lobe function. The test consists of three parts (TMT-A, TMT-B and TMT-C). In TMT-A and TMT-B, the study participant must draw lines connecting numbered circles in order and reverse-order. In TMT-C, the study participant must draw lines connecting circles alternately with letters and numbers in an ordered sequence. Time spent in TMT-C is assessed [10]. 3-SCWT. The test assesses selective attention, inhibitory ability and mental flexibility. It consists of three main components (SCWT-1, SCWT-2 and SCWT-3) using four different colours. For SCWT-1 the patient is shown a colour name word (written in black) and must say the colour name word. For SCWT-2 the patient is shown a rectangle filled with colour and the colour name word is also printed in black within the rectangle. The patient must say the colour that fills the rectangle. For SCWT-3 the patient is shown a colour name word printed in a colour different from the colour name word and they must tell the tester the colour of the printing. The name words for the colours all followed the same order as for SCWT-1. In this test, we evaluate the time spent in SCWT-3 [11].4-DST. The test assesses immediate memory. The subject is asked to listen of random numbers at a rate of one per second. In the digit span forwards (DSF), the subject repeats the numbers in a forward order, while in the digit span backwards (DSB) in reverse order. Starting with a two-number sequence, each correctly repeated series is followed by a sequence with one additional digit. For each sequence, the subject was given a second chance with another set of random numbers if he/she had failed in the first attempt. If the subject also failed in the second attempt, the test was interrupted and scored according to the longest series that was achieved. A maximum of nine digits are presented in the DSF, and eight digits in the DSB. DSF and DSB scores are summarized to get total score and no time limitations were imposed [12].5-DLST. The test measures short-term memory, visual-spatial skills and attention. The test is a task performed in 2 minutes and the study participant must write the letter referred to each number (from 1 to 9). A target of symbols and their numbers are displayed (80 symbols are provided). Number of hits and number of errors are registered [12].For the diagnosis of POCD, a composite cognitive index was established and defined by the occurrence of cognitive impairment in at least two of five possible cognitive deficits: average AVLT, SCWT-3, TMT-C, DST and DLST. The definition of cognitive dysfunction considered a reduction greater than or equal to 20% in two or more tests results compared with the preoperative assessment.
2.3. Assessment of Plasma Biomarkers
- Serum levels of glial fibrillary acidic protein (GFAP) and inrerleukin-6 (IL-6) were obtained from venous blood samples (3 ml) collected before surgery, immediately after surgery and 6 and 24 hours postoperatively. Samples were placed in dry tubes and centrifuged; serum was removed and stored at -20°C until the analysis. GFAP and IL-6 were measured by a commercially available ELISA kit (BioVendor, Czech Republic) using ELISA Microplate Reader (Hospitex Diagnostics S.R.L. Italy). GFAP and IL-6 levels were expressed as nanograms per millilitre. All samples were measured in duplicate and the coefficient of variation was less than 5% for both analyses.
2.4. Anaesthetic Management
- After admission to the operating room, patients were monitored using pulse oximetry, three-lead electrocardiogram, and capnometry (Triton Electronics, Russian Federation). Peripheral venous puncture was obtained in the upper limb with an 18 or 20G catheter. After preoxygenation with 100% oxygen in spontaneous ventilation, an intravenous administration of 3 µg/kg fentanyl followed by 2-3 mg/kg propofol (Claris Lifescience, India) was performed. Muscle relaxation was obtained with 1 to 1.5 mg/kg succinylcholine (Darnica, Ukraine) followed by tracheal intubation. Mechanical ventilation was performed in a circular valve system with carbon dioxide absorber as part of an anaesthetic machine (Chirana Venar, Slovakia). It was adjusted to a tidal volume of 6-8ml/kg, FiO2 1.0, I:E ratio of 1:1, positive end expiratory pressure of 5 cmH2O, mean respiratory rate of 12 breaths/min adjusted to maintain end-tidal carbon dioxide between 30 and 40mmHg. Anaesthesia was maintained with isoflurane (Piramal Enterprises Limited, India) adjusted between 0.5 and 1.5 minimum alveolar concentration (MAC) and intravenous fentanyl 3-6 µg/kg/h; neuromuscular block was maintained using intravenous pipecuronium bromide (Gedeon Richter, Hungary) 15-20 µg/kg/h. Each patient received multimodal postoperative analgesia using nonopioid and opioid analgesics and peripheral nerve blocks as well.
2.5. Statistical Analysis
- Categorical data are represented by absolute and relative frequencies. The numerical data are described by mean and standard deviation (SD). Variations of categorical variables were tested by Fisher’s exact test. Normality of distribution of observed numeric variables was tested by the Shapiro-Wilk test. Differences between the normal distribution of numeric variables between the two independent groups were tested by the Mann-Whitney Test or t-test in case of data are parametric or non-parametric respectively. To determine the difference between two dependent non-parametric samples, Friedman test was used. P<0.05 was considered statistically significant. Statistical analysis was performed using Microsoft Excel 2010 (Microsoft, USA) and MedCalc Statistical Software V.14.12.0 (MedCalc Software, Ostend, Belgium; http://www.medcalc.org).
3. Results
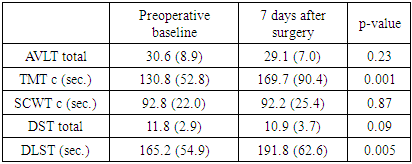
- At the 7th day after surgery with regard to overall evaluation of POCD, 13 (15.8%) patients had criteria for POCD diagnosis. The most affected cognitive domains were executive function (TMT) and short term memory (DLST) and least affected function was selective attention (Stroop’s test) (Tab. 2).
|
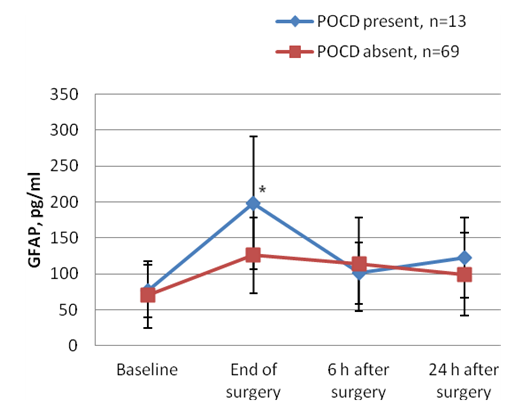
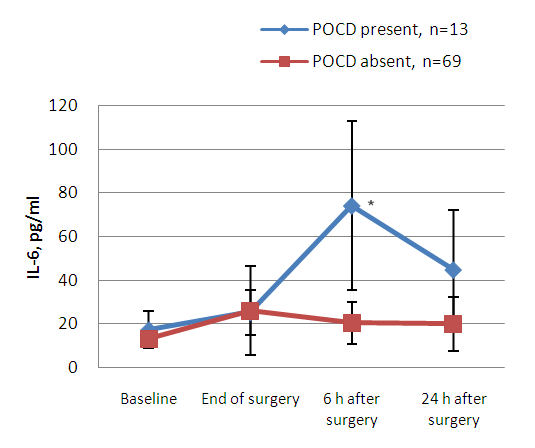 | Figure 2. Interleukin-6 (IL-6) profile regarding presence or absence of POCD 7 days after surgery. (Data are shown as mean SD. * P<0.05 compared between groups “POCD present” and “POCD absent”) |
4. Discussion
- Based on diagnostic criteria, our findings showed that the incidence of POCD 7 days after surgery was around 16%. Increased serum levels of GFAP and IL-6 in immediate and early postoperative period indicated that anaesthesia and surgery might be significantly related to a level of neural damage that correlates with POCD. Despite improvements in surgical and anaesthetic techniques over the last decades, cognitive dysfunction, with clinical manifestations, such as deterioration in memory, attention, motor function and visuospatial ability is still highly prevalent [13]. The incidence of POCD varies considerably, and after noncardiac surgery may be as high as 20 to 30% at 1 week after surgery, declining to 15% after 2 months [14].Surgery can induce an activation of the immune system, resulting in a strong systemic inflammatory response, which may cause damage to the central nervous system. When an excessive neuroinflammatory response occurs, peripherally originating cytokines can cause central neuronal and glial toxicity, thus affecting the function of synaptic connections and causing neuronal and glial toxicity. The occurrence of POCD after trauma surgery may be related to a combination of several factors often associated with microthrombus or fat embolism, severe postoperative pain and inflammatory response [15-17].In the present study, we observed that both neuropsychological tools and serum biochemical markers indicated some degree of brain damage. Both serum IL-6 and GFAP were significantly elevated after trauma surgery in patients who developed POCD and might represent systemic inflammation and glial cell damage, respectively. Considering that GFAP is highly brain specific, GFAP may be a better predictive marker of POCD. Contradictory results of different findings among authors may be related to the variability of POCD criteria, lack of comprehensive evaluation of POCD, short follow-up and/or additional pathophysiological mechanisms, involving extracerebral sources of biochemical markers. The limitations of our study were the small overall number of patients, absence of comparison of different anesthesia patients groups and the prevalence of POCD reported in previous studies varies widely and the conflicting results may be attributable to different diagnostic criteria for POCD.
5. Conclusions
- In summary, this study provides additional evidence that orthopedic surgery is associated with high postoperative serum levels of GFAP and IL-6, which may indicate significant neural damage. GFAP serum levels may be more in combination with IL-6 level for predicting POCD after orthopedic surgery. Future studies should focus on accurate detection of POCD, validation of new biochemical markers and understanding the mechanisms of POCD.
AKNOWLEDGEMENTS
- We thank Dr. Dilfuza Berdieva and Dr. Feruza Yusupova (Central Clinical Diagnostic Laboratory of Republican Research Centre of Emergency Medicine) for providing ELISA tests. Without their support this research would not be possible to done.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML