-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2020; 10(2): 27-37
doi:10.5923/j.cmd.20201002.01
Received: Sep. 4, 2020; Accepted: Sep. 28, 2020; Published: Oct. 15, 2020

A Phase I, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Tolerability of Oral Fluorescein Disodium and Confirm the Authenticity of Point of Care Urine Samples
Mark R. Dube1, Gabriel M. Theriault2, Paul L. Rheault2, Nathalie A. Roy2
1Sponsor, UPTru Inc., Sudbury, Ontario, P3E 5L7, Canada
2Research Institute, Medicor Research Inc., Sudbury, Ontario, P3A 1W8, Canada
Correspondence to: Gabriel M. Theriault, Research Institute, Medicor Research Inc., Sudbury, Ontario, P3A 1W8, Canada.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We provide further evidence that fluorescein disodium has low toxicity in humans. The safety profile of a single oral dose of 100 mg fluorescein disodium in healthy adults was consistent with the previously reported literature. There were no clinically significant differences in vitals, clinical laboratory findings, or AE incidents between baseline and 14 days post-randomization measurements. Furthermore, we show that urine sample authenticity can be ascertained using a fluorophore. The fluorescein assay was performed at four time points (10, 15, 20, and 30 minutes). The sensitivity of the assay was 100% for urine samples taken at or after 15 minutes. The results of this study further the development of fluorescent-based urine specimen identification protocols for clinical use however, future larger trials are required for greater statistical power and to optimize the assay’s cut-off time points.
Keywords: Fluorescein Disodium, Urine Drug Test, Drug Screen, Fluorescence
Cite this paper: Mark R. Dube, Gabriel M. Theriault, Paul L. Rheault, Nathalie A. Roy, A Phase I, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Tolerability of Oral Fluorescein Disodium and Confirm the Authenticity of Point of Care Urine Samples, Clinical Medicine and Diagnostics, Vol. 10 No. 2, 2020, pp. 27-37. doi: 10.5923/j.cmd.20201002.01.
Article Outline
1. Introduction
- The last decade witnessed a significant increase in the demand for urine drug testing. This increase has been fueled by the opioid crisis and the recent legalization of marijuana in several states and Canada [1–4]. The rate of workforce drug positivity (4.5%) hit a sixteen-year high in 2019 [5]. From 2011 to 2014, the spending on urine drug and related tests by Medicare and private companies quadrupled to an estimated 8.5 billion dollars (USD) [6]. Urine drug testing is a lucrative business with a single test costing between $30 and $60.The consequences of a positive urine drug test may be severe. Not surprisingly, many clients attempt to circumvent the validity of the test. Four to seven percent of urine samples submitted for drug analysis are altered in some way [1,7,8]. Common practices include urine substitution (either with synthetic or someone else’s drug-free urine), ingestion of commercial products (i.e. detox drinks or capsules) and alteration of urine post-urination using chemicals (i.e. strong oxidizers such as iodine, peroxide or peroxidase) [9–11]. Although existing laboratories can detect adulterated or synthetic urine samples, they cannot identify substitution with urine from another person. Currently, authenticity ascertainment is limited to verifying the temperature, pH, specific gravity, and creatinine levels of the urine sample [11]. Colouring the water in the toilet tank and/or shutting off the water to the restroom are techniques often used to overcome sample dilution. However, a substituted urine sample (e.g., from a concealed container) could be undetected by the latter measures. Direct observation of urination is unpalatable and humiliating for many clients, and has been taken to the supreme court of Ohio as a human rights issue [12]. Furthermore, many cultural and ethical boundaries prohibit direct observation of urination.Fluorescein disodium (FD) is a fluorophore that has an absorption and emission peak between 465 - 490 nm and 520 - 530 nm, respectively. It is a relatively non-toxic compound that is currently used in medicine (ophthalmology and optometry), hydrology (tracer dye), and forensics (microscopy and serology). Medically, it is approved by the FDA (NDA 21-980), Health Canada and the EMA for use via the parenteral route in angiography or topically for staining the cornea. Fluorescein disodium has an excellent safety profile [13]. No LD50 for fluorescein has been established in humans however, the oral LD50 for mice, rats, and rabbits is 4,738, 6,721 and 2,500 mg/kg, respectively [14–17]. The intravenous LD50 for mice, rats, rabbits and dogs is 2,000, 600 – 1,000, 300 and 1,000 mg/kg, respectively [14–17]. Multi-day dose studies have been conducted in rats and dogs. McDonald et al., [16] did not report deaths in rats receiving 1 – 400 mg/kg of intravenous fluorescein every 3 days for 4 weeks. In a canine study, deaths occurred at the 400 mg/kg dose [16]. Studies in several models have shown that fluorescein has virtually no genetic toxicity, carcinogenicity or reproductive and developmental toxicity [18–26]. In humans, the parenteral route of fluorescein administration has been investigated thoroughly however, only limited studies have been conducted with oral fluorescein. The rate of adverse events in intravenous and oral studies is under 2%, even at doses as high as 500 mg/kg [27–32]. The most common adverse events associated with oral fluorescein include minimal itching, gastric discomfort, or nausea after intake [27–32]. Anaphylactic reactions with oral, topical, and intravenous fluorescein are rare [33–37]. The standard clinical intravenous and topical dose for fluorescein is 7.1 – 10 mg/kg and 6.25 – 8.75 mg per eye, respectively [38,39].Despite its medical use for over half a century, fluorescein is presently approved by regulatory bodies in North America and Europe for intravenous or topical use only. One of our objectives in this work was to undertake the preliminary studies mandated by regulatory bodies for approval of fluorescein for oral use; a Phase I clinical trial. The current study was conducted to assess the latter requirements, i.e., the safety and tolerability of a low dose of oral fluorescein disodium. In addition, and to further our overarching goal of developing fluorescent-based urine specimen authentication protocols, we determined fluorescein disodium's fluorescent properties in post-administration urine samples. Our study provides important data for the development of new standardized urine drug testing protocols that will address the important issue of sample authenticity and facilitate better patient care and societal outcomes.
2. Materials and Methods
2.1. Oversight
- This was a Phase I, randomized, double-blind, placebo-controlled trial conducted at Medicor Research Inc. (Sudbury, Ontario, Canada) between September 2019 and November 2019. All trial-related functions (patient visits, data collection, site monitoring, and statistical analyses) were performed at this location. Biological sample measurements were performed by LifeLabs (Sudbury, Ontario, Canada). The trial was funded by UPTru Inc. The study protocol was reviewed and approved by Health Canada and an Independent Ethics Committee. The trial was conducted according to the Declaration of Helsinki and ICP-GCH. The study was registered on ClinicalTrial.gov (NCT04071080).
2.2. Study Drug
- The trial drug, fluorescein disodium (intravenous solution, FluoresciteTM) was purchased from Novartis (DIN: 00505005). Compounding was performed as per protocol by unblinded staff at Medicor Research Inc.The trial population consisted of healthy participants between the ages of 18 and 55 years old. All participants provided written informed consent prior to any study-related procedures. Adults were excluded from participating in the study if they had a history of allergy to any food or drug; including allergy to FluoresciteTM, GatoradeTM, or any of their constituents. Adults with a history of jaundice, bronchial asthma, cirrhosis, uncontrolled diabetes, renal failure, cancer, HIV, or hepatitis B/C, were pregnant or nursing were also excluded from participating in the study.
2.3. Screening, Randomization, and Follow-Up
- The trial entailed 3 visits; Screening, Randomization, and a 14-day Follow-up (FU) (Figure 1). Eligibility criteria and baseline measurements (vitals, ECG, physical, and laboratory samples) were collected during the screening visit. The screening period was 14 days to leave adequate time to receive results from the local lab. Adverse events (AE) were collected from the time of informed consent.
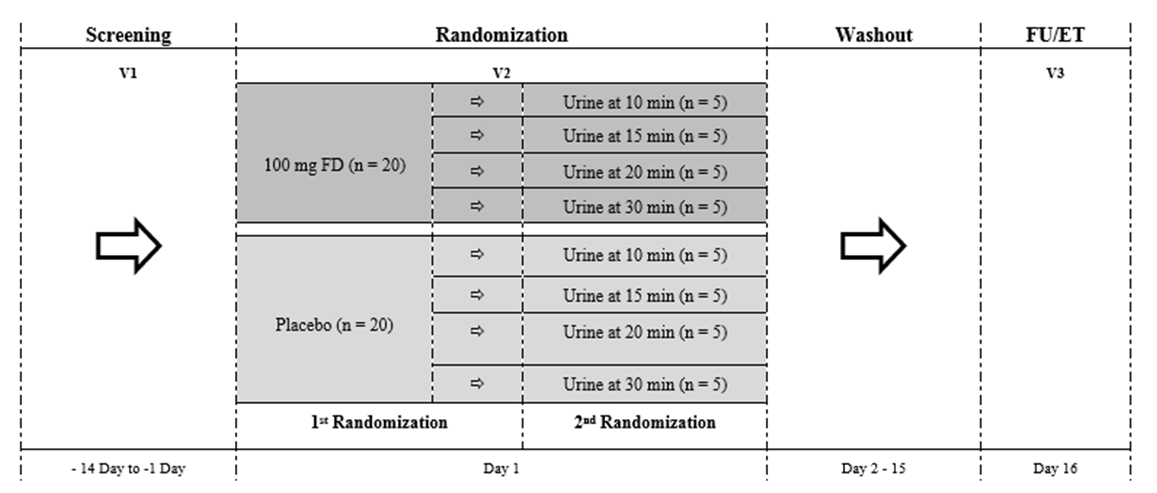 | Figure 1. Study flow chart. FU; Follow-Up, ET; Early Termination, FD, Fluorescein disodium; n, number of participants |
2.4. Statistical Analysis
- IBM SPSS Version 26 was used for all statistical analyses. An alpha of 0.05 was used to determine statistical differences.
2.4.1. Sample Size
- The present study was a first-in-human Phase I trial. The primary goal of the study was to assess the safety and tolerability of oral fluorescein disodium. The study was not designed to directly assess the efficacy of the assay however, it was constructed with the intent of estimating the minimum time point at which fluorescein fluorescence could be detected with a sensitivity and specificity of 100%. The average size of a Phase I trial is typically small (< 28 subjects), with less than 8 subjects per treatment arm [40]. Therefore, we randomized 20 subjects to each treatment group (N = 40).
2.4.2. Safety Analysis
- Only subjects who received the study drug were included in the safety analysis. A one-way between-groups ANOVA was conducted to compare the effect of the treatment on vitals, blood chemistry, blood hematology, and urine chemistry laboratory results. For urine microscopy, incidence of abnormal ECGs and incidence of AEs, Fisher’s exact test (FET) was used to determine if there was an association with treatment.
2.4.3. Sensitivity and Specificity of the Assay
- Only subjects who completed V2 were incorporated in the efficacy analysis. Subjects with major deviations (i.e. could not produce enough urine or a sample on time) were removed from the analysis. The percent of correct calls between each time point was compared using FET (alpha of 0.05). The result was deemed “incorrect” if at least one of the two observers was wrong in their assessment of the urine sample. The sensitivity and specificity of the assay were assessed by noting the presence or absence of fluorescein fluorescence in urine samples collected at four-time points (10, 15, 20, and 30 minutes) post-ingestion. Samples were marked “positive” for fluorescence if at least one of the two observers marked it positive. FET (alpha of 0.05) was used to determine statistical differences.
3. Results
3.1. Study Participants
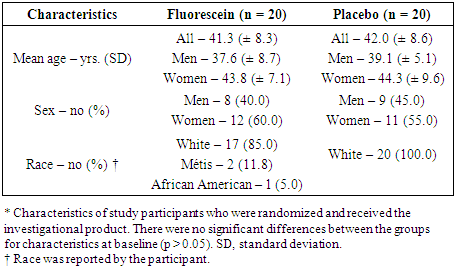
- There were 48 participants who gave informed consent, 8 did not meet eligibility criteria. In total, 40 participants were randomized in the trial (FD = 20; Placebo = 20) (Figure 1). There were no withdrawals or lost to follow-up; all 40 participants completed the study. Demographic characteristics for study participants can be found in Table 1.
|
3.2. Safety
3.2.1. Vitals and ECGs
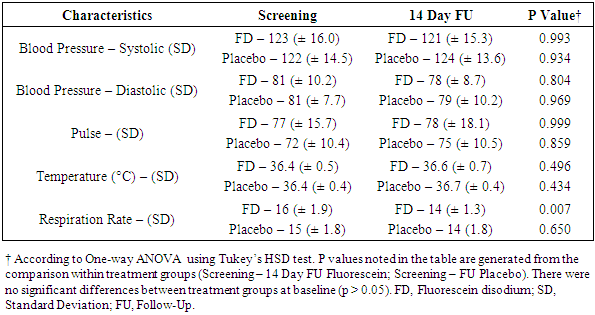
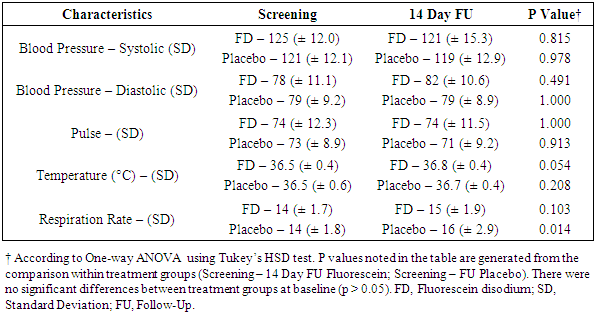
- Vitals (blood pressure, pulse, respiration rate, and temperature) were measured at four time points during the trial (screening, randomization pre-dose and 1-hour post-dose, and 14-day follow-up). The statistical analysis included a comparison between vitals obtained at the screening visit and 14-day follow-up (Table 2) as well as a separate analysis for vitals collected at the randomization visit (Table 3). The incidence of abnormal ECGs was compared between the screening visit and the 14-day follow-up.
|
|
3.2.2. Laboratory Results
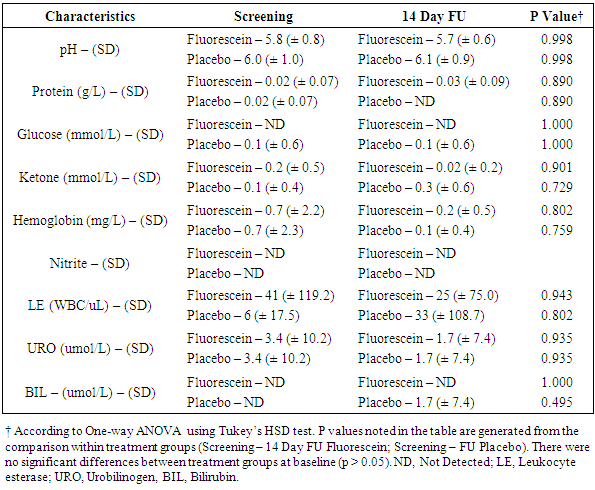
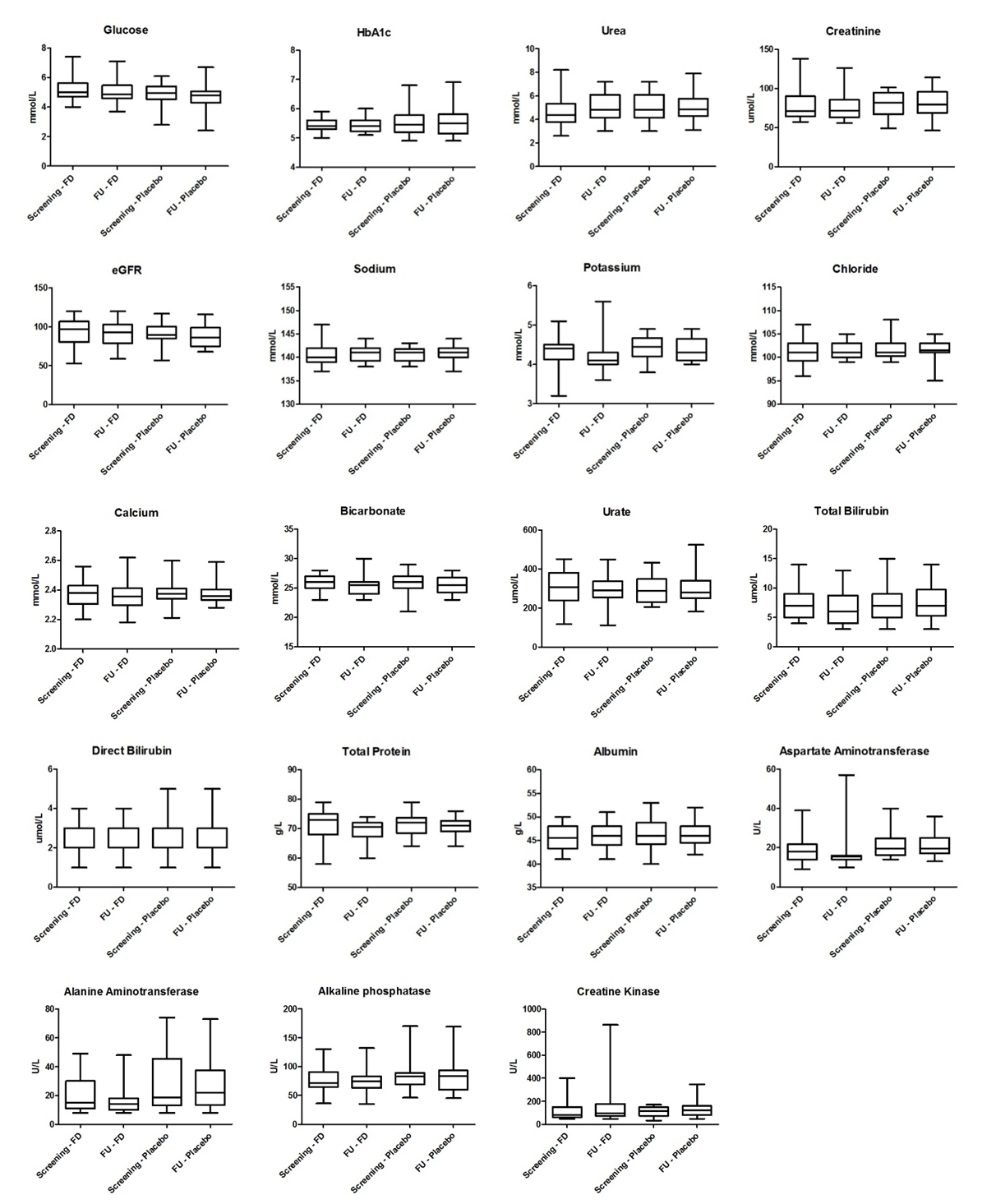
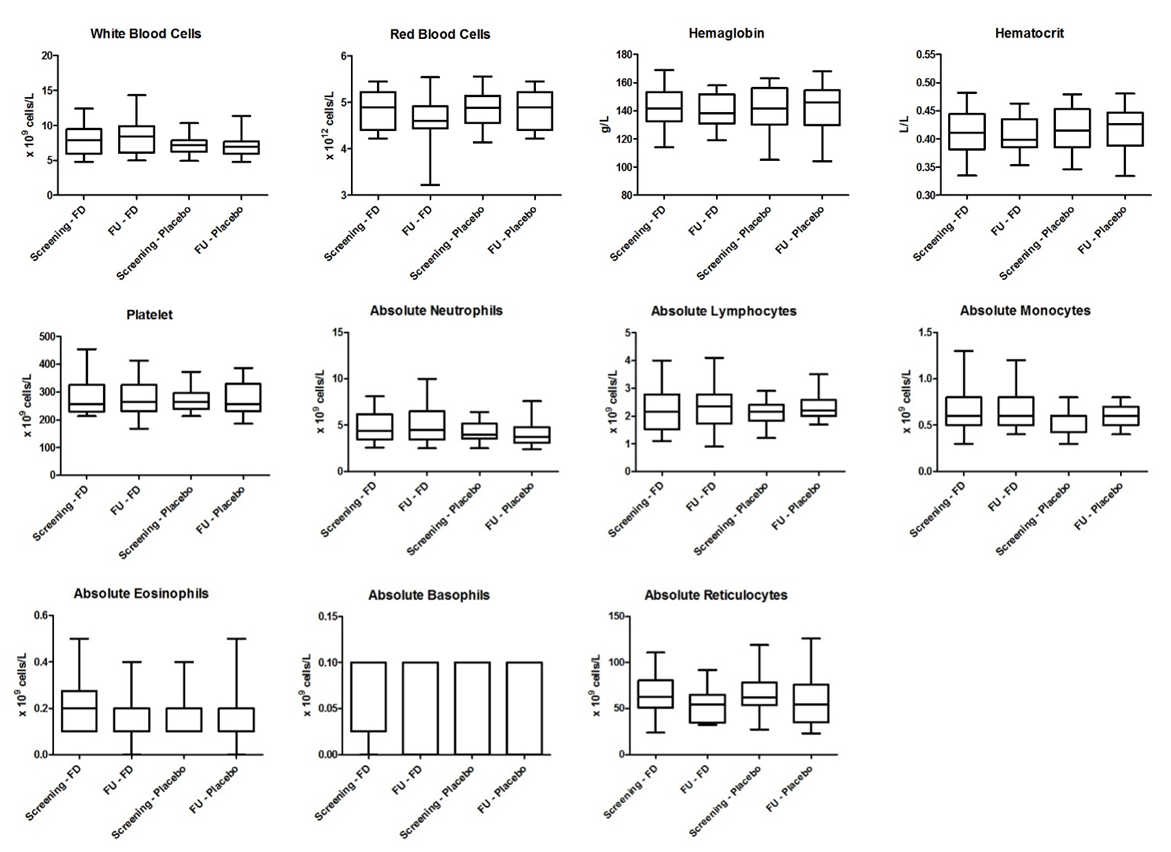
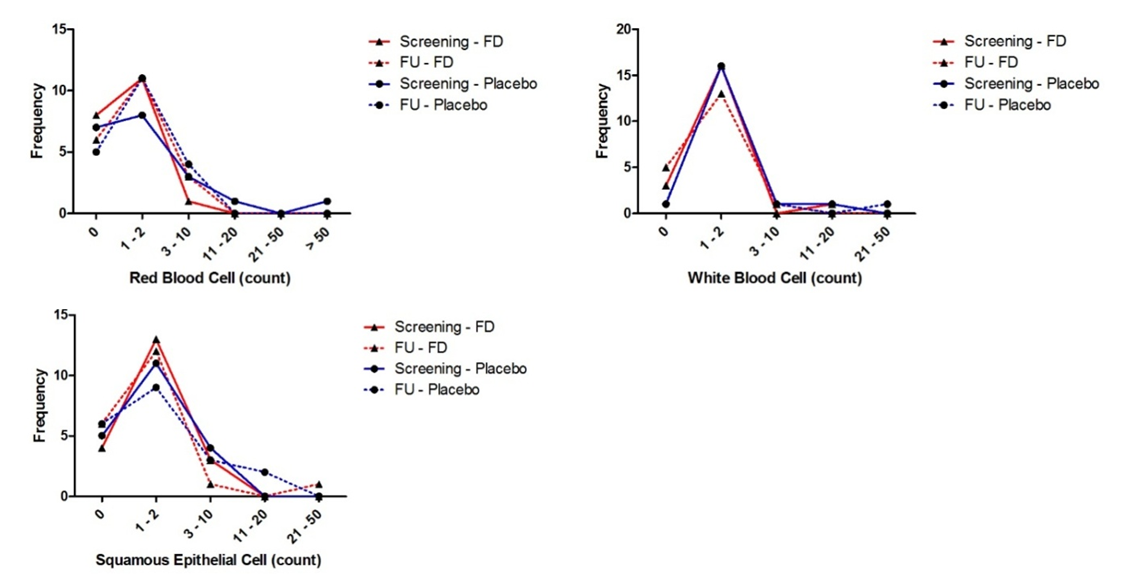
- Laboratory results for blood chemistry and hematology as well as urine chemistry and microscopy are summarised in Figures 2 to 4 and Table 4. There were no significant differences in blood chemistry and hematology measurements between the fluorescein group and the placebo group (p > 0.05). Furthermore, no significant differences were observed in chemical and microscopy urinalysis measurements between the fluorescein and the placebo group (p > 0.05).
|
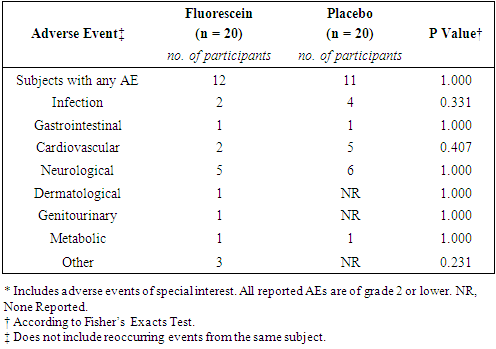
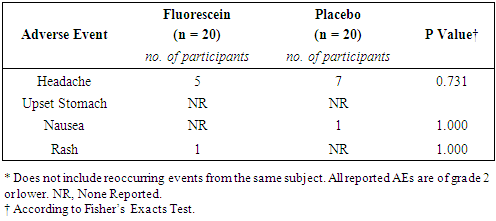
3.2.3. Adverse Events
- In total, 35 adverse events were reported (FD, n = 16; Placebo, n = 19). All adverse events were deemed not related to study drug. There were no grade 3 adverse events or serious adverse events. The rate of adverse events was similar between the two groups (Table 5).
|
|
3.3. Fluorescein Fluorescence
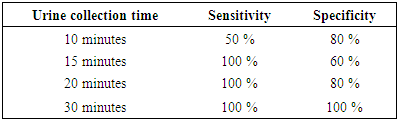
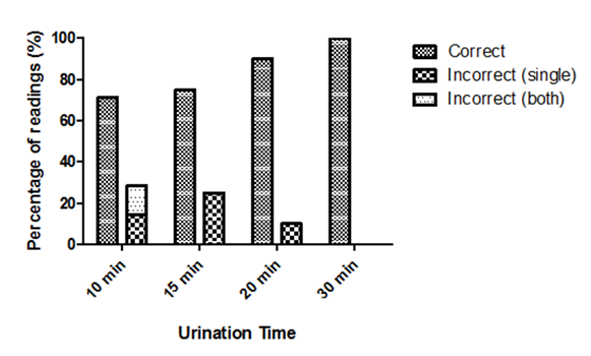
- The presence or absence of fluorescein fluorescence was observed in participant urine samples collected at specific time points (Figure 1). The percent of correct/incorrect calls made by the observers for each time point is displayed in Figure 5. The sensitivity and specificity of the assay at each time point are described in Table 7. Four subjects were removed from the analysis due to major deviations. Although not statistically significant, the general trend indicated there was a positive correlation between the urine collection time and the percentage of correct calls (p > 0.05).
|
4. Discussion
- Adulteration of urine drug testing by specimen substitution is a significant obstacle in the management of illicit drug use in clinical and workplace settings [1,6,7]. Oral administration of non-toxic compounds that fluoresce in post-ingestion urine samples may be a simple and effective approach to ensure the integrity of urine specimens for office-based urine drug testing. This was a phase I, single-center, single-dose trial in healthy adults to assess the safety of oral fluorescein and to further evaluate its utility in ensuring urine specimen authenticity. Participants in this study received 100 mg of fluorescein disodium or matching placebo orally. Overall, the safety data of the current study is consistent with the findings of previous animal and human trials. This is not surprising since 100 mg (roughly 1.3 mg/kg in an adult) is much lower than doses used clinically [38,39]. Fluorescein was well tolerated with few adverse events (none related to study drug). One subject (FD arm) did develop a mild rash (grade 1) on their neck post-dose. The event resolved the same day without any concomitant medication. Although this event was deemed not related to study drug, episodes of urticaria and rashes have been reported in 0.1 - 1% of patients taking fluorescein [28,41,42].Vitals and laboratory reports were all within normal ranges. Significant differences were observed between respiration rates. Although still within normal limits, there was a significant decrease in respiration rate 14 days post-dose in patients receiving fluorescein. There are no reported cases in the literature of decreased respiration rate in humans or animals taking fluorescein. One possible explanation could be that there were inconsistencies in measurements since an increase was observed 1-hour post-dose in participants receiving placebo. Furthermore, measurements were not always taken by the same person and it is known that respiration rate can be difficult to measure accurately due to various factors [43–45].The current trial was not specifically designed to assess the sensitivity and specificity of the assay however, it was still constructed with the intent to assess the assay's performance. Participants were asked to collect a urine sample 10, 15, 20, or 30 minutes post-dose. Although not statistically significant, we found that the ability to detect fluorescein fluorescence using an ophthalmoscope was positively correlated with the urine collection time.Several deviations were documented for participants in the 10-minute arm. Either participants were unable to provide a urine sample at the assigned time or the volume of urine was below what was required per protocol. Due to major deviations, 3/5 subjects from the fluorescein group had to be removed from the efficacy analysis. The sensitivity and specificity of the assay for the 10-minute time point was 50% and 80%, respectively. Upon intravenous administration, fluorescein can be detected via angiography within 5 minutes [46]. However, high affinity of fluorescein to proteins and rapid metabolism to glucuronide metabolites reduces its fluorescence significantly [46–48]. Participants were not asked whether or not they had emptied their bladders before arriving at the site therefore, is it possible that fluorescein was too dilute to detect in urine samples from the 10-minute group. Furthermore, both observers were unable to detect fluorescence in the two participants who provided a sample of inadequate volume (3 and 6 ml) and one participant who provided a sample per protocol. This indicates that ten minutes did not allow sufficient time for a patient to produce a urine sample large enough to detect fluorescein fluorescence with the naked eye.The sensitivity of the assay was 100% for urine samples collected at 15 and 20 minutes. However, the specificity was 60 and 80%, respectively. The specificity remained lower due to observers not agreeing on the presence or absence of fluorescein fluorescence. In fact, one of the two observers was more accurate than the other (3 vs. 6 overall incorrect calls). Even though patient samples were compared against positive and negative standards, observers had more difficulty with urine samples from the placebo group. It is possible that the natural pigmentation of urine or naturally occurring fluorophores caused some uncertainty between observers. Naturally occurring fluorophores such as flavins (i.e. vitamin B2) and vitamin B12 have similar absorption/emission peaks as fluorescein [49,50]. They could have contributed to the higher rate of false positives, especially if a participant was taking supplements. The sensitivity and specificity were 100% at the 30-minute urine collection time point. This indicates that 30 minutes should be sufficient to determine with certainty if a urine sample is authentic or not. Future studies will determine if shorter times could suffice. Additional studies with larger sample sizes are underway and will determine statistical significance and the range of acceptable cut-off time points. The use of a fluorometer is also being investigated as a means of removing the subjective aspect of the assay and potentially reducing the clinic wait time. Portable fluorometers are readily available, relatively inexpensive, and provide quantitative readings within seconds.
5. Conclusions
- Overall, the safety profile of 100 mg oral fluorescein disodium indicates that it would provide a clinically useful and robust measure of specimen authenticity. The incidence of AEs was distributed equally among participants randomized to fluorescein or placebo. Vitals and laboratory reports were all within normal ranges 1 hour and 14 days post-dose. Measuring fluorescein fluorescence within 15 minutes faces many challenges. Some of which the patient or clinic has no control over (i.e. how quickly to urinate, how much urine to produce). Furthermore, blinded observers had a difficult time differentiating urine samples with or without fluorescein. The 30-minute time point provided the greatest sensitivity and specificity. Further studies with larger sample sizes are required to statistically differentiate the time points. The use of a commercial fluorometer for the assay could provide several added benefits such as decrease wait time and higher sensitivity and specificity at lower time points.
ACKNOWLEDGEMENTS
- We would like to express our sincere appreciation and gratitude to the staff of Medicor Research Inc. for conducting the trial.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML