Amira A. Adel1, Maha A. Hassan2, Fatma M. Abd Alsalam1, Nahla M. Abd Elaziz3, Fatma M. Nasr1
1Dermatology and Venereolgy Department, Faculty of Medicine (Girls), Al-Azhar University, Cario, Egypt
2Internal Medicine Department, Faculty of Medicine (Girls), Al-Azhar University, Cario, Egypt
3Clinical Pathology Department, Faculty of Medicine (Girls), Al-Azhar University, Cario, Egypt
Correspondence to: Maha A. Hassan, Internal Medicine Department, Faculty of Medicine (Girls), Al-Azhar University, Cario, Egypt.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Uremic pruritus (UP) is a common and burdened symptom for patients with End Stage Renal Disease (ESRD) affecting up to 46% of hemodialysis patients. The pathophysiology of uremic pruritus is not well known and is multifactorial. Aim of the study: To evaluate soluble Erythropoietin receptor in the serum of HD patients, and to clarify its possible role in pathogenesis of pruritus and correlate its level with the severity of itching in HD patients. Subjects and methods: Across section case control study was carried out on sixty ESRD patients on regular HD, thirty patients with UP group (A) and thirty patients without UP group (B), and thirty apparently healthy age and sex matched subjects as control. Assessment of itching severity by 5-D itch scale (degree, duration, direction, disability and distribution). Serum Urea, Creatinine, Calcium, Phosphorus and Parathyroid Hormone levels, Serum (s-EPOR) were measured in both patients and healthy controls. Results: There was significant increase of s-EPOR level in serum of HD patients in comparison with control group and significant increase of its level in patients with pruritus more than patients without pruritus. The Severity of pruritus according to 5-D scale in group (A) was (50%) mild, (43.3%) moderate and (6.7%) severe. There were no significant relation between s-EPOR level and severity of pruritus, duration of dialysis, or pruritus. Conclusion: The study concluded that elevated serum level of soluble erythropoietin receptor in HD patients with pruritus more than those without pruritus.
Keywords:
ESRD, UP, HD, s-EPOR
Cite this paper: Amira A. Adel, Maha A. Hassan, Fatma M. Abd Alsalam, Nahla M. Abd Elaziz, Fatma M. Nasr, Evaluation of Soluble Erythropoietin Receptors in Hemodialysis Patients with and without Pruritus, Clinical Medicine and Diagnostics, Vol. 10 No. 1, 2020, pp. 20-25. doi: 10.5923/j.cmd.20201001.03.
1. Introduction
Pruritus is considerd a highly prevalent condition in advanced chronic kidney disease (CKD) and ESRD patients on regular dialysis [1]. The pathophysiology of UP is complex and involves multiple assumption mechanisms contributing to pruritus including anemia, inadequate dialysis, histamine release from skin mast cells, skin dryness and secondary hyperparathyroidism as well as high B2-microglobulin. Hyperparathyroidism can stimulate mast cells to release histamine and can promote micro precipitation of calcium and magnesium salts in the skin [2]. The relationship between somatic neuropathy and UP and or dysautonomia were also suggested [3]. It was proposed that the itch associated with CKD is a manifestation of an immune system derangement with overactivation of CD4+ TH1 lymphocytes [4].Erythropoietin is a growth factor commonly used to manage anemia in patients with CKD, it acts through the erythropoietin receptor (Epo R) present in erythroblasts [5]. Alternative mRNA splicing produces a soluble form of EpoR found in human blood. EPO modifies the cellular inflammation process by inhibiting the expression of pro-inflammatory cytokines IL-1 and TNF-α and decreased pro-inflammatory mediators such as osteopontin and C-reactive protein [5]. Khankin et al [6] suggested that circulating sEpoR competes with erythropoietin for receptor binding and that elevated levels of sEpoR at initiation of hemodialysis portend increased erythropoietin dose requirements needed to sustain target hemoglobin levels. Furthermore they hypothesized that sEpoR production may be mediated by inflammatory cytokines present in the uremic milieu. Aim of the study: To evaluate soluble Erythropoietin receptor in the serum of HD patients, and to clarify its possible role in pathogenesis of pruritus and correlate its level with the severity of itching in HD patients.
2. Subjects and Methods
A cross section case control study was carried out on sixty patients on HD three times per weekly, thirty patients with UP and thirty patients without UP and thirty apparently healthy age and sex matched subjects as controls. All subjects ages ranged from 19 to 60 years mean ±SD (33.9±10.96). ESRD patients on regular HD for more than 6 months, their age more than 18 years were included in the study, all Patients were recruited from the haemodialysis unit of AL Zahraa University Hospital in Cairo, Egypt, over a period of twelve months (January 2015- December 2016). All the patients were dialyzed three times a week for 4 h were using high flux dialyzer with reverse osmosis purified water and bicarbonate containing dialysate. Polysulfone membranes were used. Bloodlines and filters were steam-sterilized. Moreover, patients had to be adequately dialyzed with a single Kt/V of at least 1.2.After explaining the purpose of the study which was approved by the faculty ethics committee and taking the consents of both cases and controls; data were collected through personal interview and they were asked to fulfill a specially designed structured questionnaire. We have excluded patients with Systemic pruritic diseases such as hepatic disease and allergic diseases, Pruritic dermatologic diseases as various forms of eczema, lichen simplex, urticaria and infections, patients with immunosuppressive disease as HIV infection and neoplastic diseases, patients on immunosuppressive therapy in the past 3 months or erythropoietin therapy.All patients were subjected to the following: Full medical history with special reference to age, sex, duration of dialysis, duration and intensity of pruritus. General and local examination including chest, heart, abdomen, neurological and dermatological examination for exclusion of other causes of pruritus.Assessment of itching severity by 5-D itch scale which is multidimensional questionnaire containing five dimensions (degree, duration, direction, disability and distribution) designed to be useful as an outcome measure for itching severity in clinical studies [7].The scores of each of the five domains were achieved separately and then summed together to obtain a total 5-D score. 5-D scores can potentially range between 5 (no pruritus) and 25 (most severe pruritus) [7].Laboratory tests for all studied groups including Serum Urea, Creatinine, Calcium (Ca+), Phosphorus (p) and Parathyroid Hormone (PTH) levels were done using (Cobas C311, Roche, Germany). Serum (s-EPOR) by an Enzyme linked immunosorbent assay (ELISA) kit.. GLORY SCIENCE CO., LTD Address 2400 veterans Blvd.Suite 16-101 Del Rio, Tx 78840, USA.Five ml of venous blood were aseptically collected from each patient and control. Samples were dispensed in tubes and left to clot for 30 minutes at 37°C, then they were centrifuged at 2000-3000 R/m for 20- minutes. The collected sera were finally stored at -80°C until analysis.
3. Elisa: Principle of the Assay
Patient's serum was added (contain EPOR antigens) to microliters plate wells coated with EPOR antibodies to form EPOR- EPOR antibodies- enzyme complex with HRP labeled enzyme. After washing, TMP substrate was added which became blue at HRP enzyme catalyzed, the reaction was terminated by the addition of sulphuric acid. The optical densities (OD) of the samples were measured spectrophotometrically at wave length 450 nm; the concentration of EPOR in the samples is then determined by comparing the OD of the samples to the standard curve.Statistical analysis: Data were analyzed using Statistical Program for Social Science (SPSS) version 18.0. Quantitative data were expressed as mean± standard deviation (SD). Qualitative data were expressed as frequency and percentage. The following tests were done:Independent-samples t-test of significance was used when comparing between two means. Chi-square (X2) test of significance was used in order to compare proportions between two qualitative parameters. Pearson's correlation coefficient (r) test was used for correlating data. Probability (P-value), the Level of significance was set as P-value <0.05.
4. Results
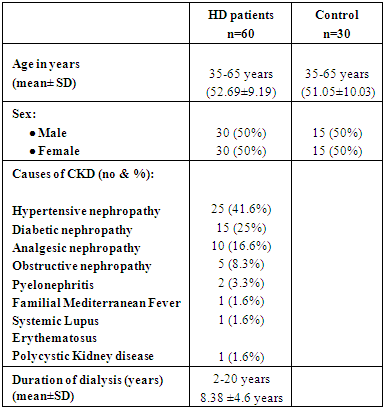
This study was carried out on sixty ESRD patients on HD, thirty patients with UP group (A) and thirty patients without UP and group (B). They were thirty males (50%) and thirty females (50%); their ages ranged from 35 to 65 years mean ±SD (52.69±9.19). They were recruited from HD unit of AL- Zahraa University Hospital. Thirty healthy volunteers of age and sex matched were chosen as controls. Demographic, clinical features, causes of CKD showed in (Table 1).Table (1). Demographic, clinical features in studied patients and control group
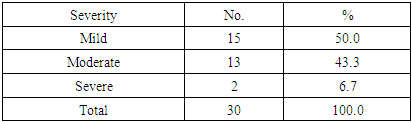
 |
| |
|
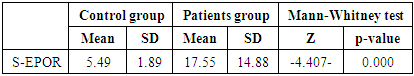
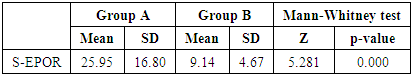
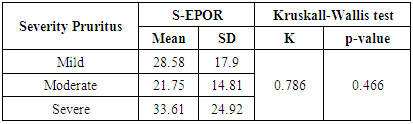
In this study there was a highly statistically significant increase in s-EPOR level in HD patients with mean ±SD (17.55±14.88) as compared to control group (5.49±1.89), (P <0.01) (Table 2), (Fig 1). Also there was statistically significant increase in s-EPOR level in patients with pruritus (group A) with mean ±SD (25.95±16.80) as compared to patients without pruritus group (B) (9.14± 4.67), (P <0.01). (table 3). (Fig 2). Severity of pruritus according to 5-scale of itching severity in group (A) was divided into mild (50%), moderate (43.3%), severe (6.7%) (Table 4). As regards severity of pruritus, there was no statistically significant relation between s-EPOR level and severity of pruritus (P >0.05), (Table 5). There was no correlation found between s-EPOR with duration of pruritus and duration of dialysis in group (A). (Table 6).Table (2). Comparison between patients and control groups regarding S-EPOR
 |
| |
|
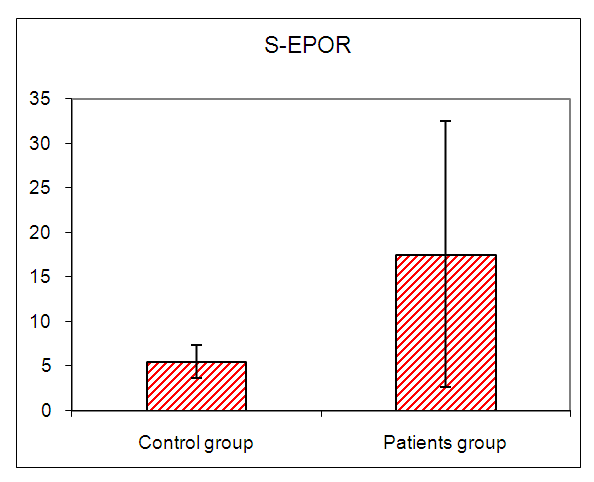 | Figure (1). Comparison between patients and control groups regarding S-EPOR |
Table (3). Comparison between group A and group B regarding S-EPOR
 |
| |
|
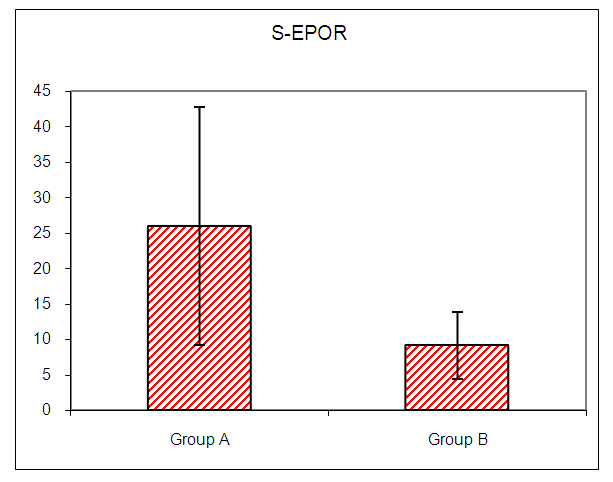 | Figure (2). Comparison between Group A and Group B regarding S-EPOR |
Table (4). Severity distribution of the pruritus
 |
| |
|
Table (5). Relation between S-EPOR and severity of pruritus in group A
 |
| |
|
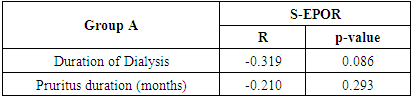
Table (6). Correlation between S-EPOR with duration of dialysis, duration of pruritus in group A
 |
| |
|
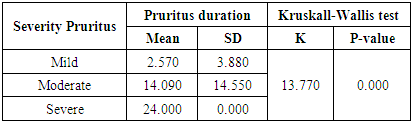
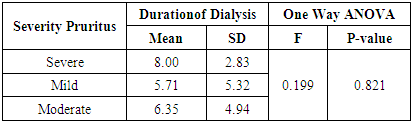
There was increase duration of pruritus in severe pruritus with mean ±SD (24.00±0.00) when compared to moderate and mild pruritus (14.090±14.550), (2.570±3.880) respectively. Relation between severity of pruritus (which is dignosed by 5-d scale) and duration of pruritus was statistically significant (p <0.01). (Table 7). While there was no statistically significant difference between severity of pruritus and duration of dialysis (p >0.05), (Table 8).Table (7). Relation between severity of pruritus and pruritus duration
 |
| |
|
Table (8). Relation between severity of pruritus and duration of dialysis
 |
| |
|
There were no significant correlation found between s-EPOR level and levels of serum urea, creatinine, ca+, Ph and PTH in group A or group B,. Also there were no statistically significant relation between severity of pruritus and serum ca+, Ph and PTH in patients of group (A), (p >0.05).
5. Discussion
All evidence proved that Uremic pruritus or chronic kidney disease-associated pruritus (CKD-aP) remains one of the most distressing and probably disabling symptoms in patients with ESRD [8]. It affects 25% of patients with CKD and 50%-90% of patients on dialysis [9].The main hypotheses that were proposed to explain the underlying pathophysiological mechanisms of uremic pruritus are the immune hypothesis [10] and the opioid hypothesis derangements and microinflammation as possible causes of CKD-aP [8].Firstly, the immune hypothesis considered uremic pruritus to be an inflammatory systemic disease with overactivation of CD4 TH1 lymphocytes with overproduction of IL-2, IFN-γ and TNF-α. [11]. There is no doubt that the increased serum levels of inflammatory biomarkers such as C-reactive protein and IL-6, confirms the inflammatory nature of the disease [4]. Furthermore, proteases are pruritogenic through activation of the distal ends of fibers C, and release of substance P, which in turn binds to NK1 receptors in mast cells leading to increased production of TNF-α [12].Erythropoietin receptors (EpoRs) are found in a variety of locations, including glomerular, mesangial, and tubular epithelial cells [13]. The intracellular domain of the EpoR contains phosphotyrosines that activate certain molecular cascades, including that involving the protein kinase B pathway, which also stimulates NF-kB activation [13].The EpoR isoforms with non-erythropoietic effects, such as tissue protection, have a lower affinity for EPO binding [14]. Therefore, the EPO dose used for this purpose should be greater than is that used in order to achieve erythropoietin effects [13].According to several studies and trials, it appears that UP is a systemic inflammatory disease rather than a local dermatologic disorder. Derangement of TH cells balance with TH1 predominance appears to be a major contributor to this systemic inflammation [4]. TH1 cells produce inflammatory cytokines such as IFN-γ that recruit and activate leukocytes; therefore, the overactivity of TH1 cells results in an inflammatory response [4].In fact, pro-inflammatory cytokines were shown to trigger the suppression of renal erythropoietin production and therefore erythropoiesis. Inhibition of Epo production was shown in vitro and in vivo to potentially involve IFN, IL-1 and -6, and TNF [15].It is interesting to investigate whether there is any correlation between uremic pruritus and the sEPOR concentration.The hypothesis was to find an association between sEPOR level and pathogenesis of uremic pruritus aiming to understand the pathways leading to chronic inflammatory state in UP. That may help finding novel anti-Prurituc drugs.The current study was carried out on 60 patients on maintenance haemodialysis divided into two groups: group (A) consisted o f 30 patients with UP, group (B) consisted of 30 patients without UP and Thirty healthy volunteers were chosen as controls. All studied groups were assisted for s EPOR.The current study showed that there was statistically significant increase in s EPOR level in patient groups in comparison with control group.This in agreement with Inrig et al [16] who postulated that circulating plasma s EpoR levels may be relatively elevated in CKD patients requiring high doses of erythropoietin-stimulating agents (ESA) for the treatment of anemia and found that higher ESA dose requirement was correlated with higher levels of circulating s EpoR.Also Khankin et al [6] demonstrated that sEpoR is present in the serum of patients at the initiation of hemodialysis and explained the mechanism for elevated sEpoR by showing that IL-6 and TNF-α can increase sEpoR production in the supernatant of cell lines expressing endogenous EpoR. They also measured circulating levels of IL-6 in a subset of patients with high sEpoR and low sEpoR and found that IL-6 levels were on average 2.5 times higher in subjects with high circulating levels of sEpoR than in subjects with low sEpoR levels.In the current study there was statistically significant increase in sEPOR level among patients with pruritus (group A) in comparison with patients without pruritus (group B). While there was no significant association between duration of dialysis, duration of pruritus, severity of pruritus, laboratory data of group A and sEPOR level.This explained by Kimmel et al [17] who indicate that an up-regulated inflammatory state is associated with UP in haemodialysis patients. In the absence of clinical signs of inflammation, elevated pro-inflammatory cytokines as IL6, TNF-α and serum acute phase proteins. This supported by Szepietowski et al [18] who showed the successful use of narrow band UVB therapy (NB-UVB) in the treatment of uremic pruritus. The NB-UVB therapy supposedly depleted the mast cell in the uremic skin to reduce the itching. This augmented the hypothesis that release of cytokines initiates uremic pruritus.This also supported by Resic et al [19] who continuously applied EPO for the three years and noticed an improvement in the subjective symptoms and less marked pruritus.This also agree with Inrig et al [16] who demonstrated that patients requiring high-dose ESA for treatment of CKD-related anemia are more likely to have increased levels of the pro-inflammatory biomarkers IL-6 and CRP.This agree with Tanaka et al [20] who showed that there was evidence of a relationship between the serum level of CRP, a marker of chronic inflammation, and cardiovascular disease in CKD patients and the use of ESA showed an independent inverse correlation with CRP. These findings imply that use of ESA in the predialysis phase of CKD has an anti-inflammatory effect and would be beneficial for prevention of progression of atherosclerotic complications.The relation between EPO hormone and s EPOR is inverse relationshipThis agree with Khankin et al [6] who found that circulating sEpoR competes with erythropoietin for receptor binding and that elevated levels of sEpoR at initiation of hemodialysis portend increased erythropoietin dose requirements needed to sustain target hemoglobin levels.This also in agreement with Brugniaux et al [21] who demonstrated in humans that plasma s EPOR concentration is decreased during exposure to Intermittent Hypoxia and observed an increase in EPO concentration concomitant with the decrease in s EPOR.In our study according to 5-scale of itching severity, the intensity of itching in group (A) was mild, moderate and severe, in 15 (50%), 13 (43.3%) and 2 (6.7%) of patients, respectively. This agree with Resicet al [19] which study included 77 patients, pruritus was found in 45 patients (58, 44%), severe in 17 (78%), moderate in 40 (0%) and mild in 42 (2%) of patients respectively. In the current study there was no statistically significant difference between severity of pruritus and duration of dialysis this agrees with Gatmiri, et al. [22] who found that there is no correlation of pruritus intensity with duration of dialysis.In this study there was no significant relation between severity of pruritus and serum levels of ca+, P and PTH in patients of group (A). This agreed with Melo et al [23] who did not find any impact from calcium, PTH and phosphate on pruritus symptom.But Gatmiri et al [22] found phosphate level but not PTH correlated with the complaint of pruritus in hemodialysis patients. It is in sharp contrast with Jamal and Subramanian studies [24] who found higher PTH level in those with pruritus However; they did not find correlation with calcium.
6. Conclusions
Serum level of s EPOR was higher in HD patients with pruritus more than those without pruritus but no relation between its level and severity of pruritus in HD patients.
7. Recommendations
Further studies are recommended to test the use & efficacy of anti- soluble erythropoietin receptor in treatment of uremic pruritus. Additional studies to evaluate membranous erythropoietin receptor in tissue sample in addition to soluble form in blood sample.
References
| [1] | Shayan S, Olufemi A, Youngjun P, Nawsheen C, Kathleen L, Linle H, Nobuyuki MandVandana S Mathur. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017, 10: 11–26. |
| [2] | Shirazian S, Kline M, Sakhiya V, Schanler M, Moledina D, Patel C, Hazzan A, Fishbane S. Longitudinal predictors of uremic pruritus. J Ren Nutr. 2013, 23(6): 428-431. |
| [3] | Mathur VS, Lindberg J, Germain M, Block G, Tumlin J, Smith M, Grewal M, McGuire D, ITCH National Registry Investigators. A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol. 2010, 5(8): 1410-9. |
| [4] | Mohammad K F, Jamshid R, Bita G, Mohammad R N. Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrology Dialysis Transplantation. 2011, 26(10): 3338–3344. |
| [5] | Gabriel H W, Enrique V, Vaughn A B, Lorna GM, and Colleen G J. Erythropoietin and Soluble Erythropoietin Receptor: A Role for Maternal Vascular Adaptation to High-Altitude Pregnancy J Clin Endocrinol Metab. 2017; 102(1): 242–250. |
| [6] | Khankin EV, Mutter WP, Tamez H, et al. Soluble Erythropoietin Receptor contributes to Erythropoietin Resistance in End-stage Renal Disease. PLOS ONE., 2010; 5 (2): e9246-9256. |
| [7] | Elman S, Hynan LS, Gabriel V, et al. The 5-D itch scale: a new measure of pruritus. Br. J. Dermatol. 2010; 162 (3): 587-593. |
| [8] | Thomas M and Andreas E K.Uremic pruritus. Kidney International. 2015, 87(4): 685–691. |
| [9] | Enas ASA and Ahmed A H.Uremic pruritus pathogenesis. Arab Journal of Nephrology and Transplantation 2014, 7(2): 91-96. |
| [10] | Rashid Dar N and Akhter A. Clinical characteristics of uremic pruritus in patients undergoing haemodialysis. J Coll Physicians Surg Pak., 2006; 16: 94-96. |
| [11] | Chen YC, Chiu WT, Wu MS. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am J Kidney Dis, 2006; 48 (1): 69-76. |
| [12] | Kfoury L and Jurdi M. Uremic pruritus. J Nephrol. 2012; 25 (05): 644-652. |
| [13] | Johnson DW, Vesey D and Gobe GC. Erythropoietin protects against acute kidney injury and failure. Open Drug Disco J., 2010; 2: 8–17. |
| [14] | Moore and Bellomo. Erythropoietin (EPO) in acute kidney injury. Annals of Intensive Care. 2011; 1: 3. |
| [15] | Buck I, Morceau F, Cristofanon S, et al., “Tumor necrosis factor α inhibits erythroid differentiation in human erythropoietin-dependent cells involving p38 MAPK pathway, GATA-1 and FOG- downregulation and GATA-2 upregulation,” Biochemical Pharmacology., 2008; 76, (10): 1229–1239. |
| [16] | Inrig J, Bryskin S, Patel U, et al. Association between high-dose erythropoiesis stimulating agents, inflammatory biomarkers, and soluble erythropoietin receptors. BMC Nephrology. 2011; 12: 67-80. |
| [17] | Kimmel M, Alscher DM, Dunst R, et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant., 2006; 21: 749–55. |
| [18] | Szepietowski JC, Sikora M, Kusztal M, et al. Uremic pruritus: a clinical study of maintenance hemodialysis patients. J Dermatol 2002; 29: 621–627. |
| [19] | Resic H, Alendar F, Kukavica N, et al. Uremic Pruritus in Haemodialysis Patients. BANTAO J. 2007; 5 (1): 19-22. |
| [20] | Tanaka Y, Joki N, Hase H, et al. Effect of erythropoietin-stimulating agent on uremic inflammation. J Inflammation. 2012; 9: 1-17. |
| [21] | Brugniaux J.V, Pialoux v, Foster G, et al. Effects of intermittent hypoxia on erythropoietin, soluble erythropoietin receptor and ventilation in humans", Eur Respir J., 2011; 37: 880–887. |
| [22] | Gatmiri S, Mahdavi-Mazdeh M, Lessan-Pezeshki M, et al. Uremic Pruritus and Serum Phosphorus Level. Acta Medica Iranica., 2013; 51 (7): 477-481. |
| [23] | Melo NC, Elias RM, Castro MC, et al. Pruritus in hemodialysis patients: the problem remains. Hemodial Int 2009; 13 (1): 38-42. |
| [24] | Jamal A and Subramanian PT. Pruritus among end-stage renal failure Patients on Hemodialysis. Saudi J Kidney Dis Transpl., 2000; 11 (2): 181-185. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML






