-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2019; 9(4): 79-88
doi:10.5923/j.cmd.20190904.04

Frequency of CD19+ CD5+ B lymphocytes among Egyptian Patients with Rheumatoid Arthritis and Their Correlation with Disease Activity and Bone Resorption
Asmaa S. Hassan1, Abeer M. Abdul-Mohymen1, Eman S. Albeltagy2, Doaa R. Amin3, Asmaa M. Elnaser4
1Clinical Pathology Department, Faculty of Medicine (For Girls), Al-Azhar University, Egypt
2Internal Medicine Department, Faculty of Medicine (For Girls), Al-Azhar University, Egypt
3Biochemistry Department, Faculty of Medicine (For Girls), Al-Azhar University, Egypt
4Medical Microbiology & Immunology, Faculty of Medicine (For Girls), Al-Azhar University, Egypt
Correspondence to: Asmaa S. Hassan, Clinical Pathology Department, Faculty of Medicine (For Girls), Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: The mechanism of osteoporosis in rheumatoid arthritis (RA) is multifactorial. Autoimmunity and chronic inflammation, among pathogenic factors, are closely related to bone resorption. However, the contribution of B cells to bone resorption in patients with RA is still controversial. Aim: This study aimed to assess the expression of CD19+CD5+ lymphocytes frequency in the peripheral blood of RA patients and their correlation with disease activity, bone resorption and bone density to determine whether this cell has a role in RA and its related bone loss. Subjects and methods: A prospective cross-sectional observational study was carried out on 34 RA patients fulfilling the ACR/EULAR (2010) criteria and 20 healthy age and sex matched healthy controls. Blood was analyzed using flow cytometry to determine the percentage of B lymphocytes expressing cell surface markers CD19+ CD5+. Rheumatoid factor (RF), anti-citrullinated protein antibody (Anti CCP) and bone resorption marker C-terminal telopeptide of type I collagen (CTX-1) were determined from the patients’ sera. Disease activity was assessed by disease activity score (DAS28-ESR) and bone mineral density (BMD) was determines by Dual Energy X-ray Absorptiometry (DEXA). Results: The percentages of circulating CD19+ CD5+ B lymphocytes were significantly higher in RA patients compared to healthy controls (1.60 % Vs 0.90% respectively; P = 0.002) and in RA patients with high activity compared to those with low activity (p=0.013). The percentages of circulating CD19+ CD5+ B lymphocytes in RA patients was positively correlated to ESR (r = 0,449, P = 0.008), DAS28-ESR (r = 0.515, P = 0.002), and RF (r = 0.389, P=0.023) and negatively correlated with BMD at left forearm (r = -0.573, P = 0.000) but not at lumbar spine, total left femur or bone resorption marker (CTX-1) in RA patients. Conclusion: The frequency of CD19+CD5+ B lymphocyte were higher in Egyptian RA patients compared to normal controls and correlates positively with disease activity parameters and inversely with BMD measurement at forearm site but not at lumbar spine, femur or bone resorption marker (CTX-1) in RA patients suggests a possible role of CD19+CD5+B lymphocyte in RA pathogenesis. However, it's weakly associated with RA related bone loss and for better clarification of the role of CD19+CD5+ B lymphocyte on bone mass in RA, larger scale longitudinal are recommended.
Keywords: Rheumatoid arthritis, CD19+ CD5+ B lymphocyte, Bone turnover marker, Bone Mineral density, Disease activity
Cite this paper: Asmaa S. Hassan, Abeer M. Abdul-Mohymen, Eman S. Albeltagy, Doaa R. Amin, Asmaa M. Elnaser, Frequency of CD19+ CD5+ B lymphocytes among Egyptian Patients with Rheumatoid Arthritis and Their Correlation with Disease Activity and Bone Resorption, Clinical Medicine and Diagnostics, Vol. 9 No. 4, 2019, pp. 79-88. doi: 10.5923/j.cmd.20190904.04.
Article Outline
1. Introduction
- Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammatory arthritis and extra-articular manifestations [1]. Cells of the innate and adaptive immune systems infiltrate the synovial membrane and forming highly aggressive pannus tissue responsible for joint destruction and local bone erosion. However, generalized bone loss with reduced bone mass was established as an extra-articular complication of RA [2]. Patients with RA have an annual decline of 3.9% and 2.5% of bone mineral density (BMD) at the lumbar spine and femoral neck sites respectively [3]. Furthermore, the decreased BMD at the forearm correlates negatively with disease activity and markers of bone resorption [4]. The bone erosions can be attributed to increase in bone resorption activity mediated by bone-resorbing osteoclasts and caused mainly by sustained inflammation [5]. Inflammatory cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), IL-1, and IL-17 can promote osteoclasts activation and bone resorption in RA patients. Many factors may also contribute as disease activity, glucocorticoid use, female gender, older age, and decreased mobility [6].The interaction between the immune and skeletal systems was evident and focused in osteoimmunology research field [7]. B lymphocytes, as a key player in humeral immunity, are one of the most important cell types in osteoimmunology. However, the contribution of B cells to bone erosion in RA is a matter of debate [8]. Some authors have demonstrated that B cells enhance osteoclastogenesis via expression of receptor activator of nuclear factor kB ligand (RANKL) [9] or via up regulation of CD5+ B cells that produce IL-6 to promote osteoclast activation [10]. Conversely, others have shown that B cells inhibit osteoclastogenesis by secretion of TGF- β [11] or by regulatory B cells characterized by the expression of IL-10 [12].CD19+CD5+ lymphocytes are subpopulation of B lymphocyte that arises early in life and responsible to produce natural antibodies with low-affinity and self-reactivity. It plays an important role in the pathogenesis and progression of various autoimmune disorders through autoantibody production, activation of the autoreactive CD4 T cells by its potent antigen-presenting activity, promotion of Th1 and Th17 differentiation and inhibition of T regular cell differentiation as well as cytokine production [13].The contribution of CD19+ CD5+ B lymphocyte in bone resorption and its relation to disease activity in RA patients is still limited. A cross sectional observational study was designed to assess the contribution of CD19+CD5+ B lymphocytes in the process of bone resorption in RA by;1) asses the frequency of expression of CD19+CD5+ B lymphocytes in the peripheral blood of RA patients and its correlation with disease activity using disease activity score (DAS28-ESR); 2) study the correlation between CD19+CD5+ B lymphocytes and parameters of bone resorption by using CTX-1, generated during the degradation of collagen type I via cathepsin K, as a serum marker for ongoing bone turnover and bone mineral density (BMD) by dual-energy X-ray absorptiometry (DEXA) as a diagnostic mean for osteoporosis in those patients.
2. Subjects and Methods
2.1. Study Design
- This is a prospective cross-sectional observational study. The subjects included were 34 Adult RA patients, fulfilled the American College of Rheumatology (ACR) classification criteria 2010 /European League against Rheumatism (EULAR) criteria for the diagnosis of RA [14] and 20 age and sex matched healthy controls. The patients were recruited from in-patient department and out-patient clinic of rheumatology unit in internal medicine department of Al-Zahraa University hospital, Cairo, Egypt during the period between November 2018 and April 2019. Informed consent was obtained from all study participants in advance. All procedures were performed in accordance with the guidelines in the Declaration of Helsinki and approved by Research Ethics Committee of Faculty of Medicine for Girls, Al-Azhar University.Exclusion criteria were history of other autoimmune diseases, metabolic bone diseases or immobility, diabetes mellitus, chronic hepatitis, primary kidney disease, malignancy, postmenopausal women, pregnancy, breast –feeding, and drugs that might alter bone metabolism as vitamin D, hormone replacement therapy or corticosteroid at a dose of more than 10 mg/day.All patients were subjected to a standardized questionnaire including: age, sex, duration of RA, systemic manifestations, and medications received. Physical examination was done with special emphasis to resting blood pressure, height, weight. Body mass index (BMI) was calculated as weight/height 2 (kg/m2). Disease activity was assessed by disease activity score (DAS-28) [15] that determining the activity through the number of swollen and tender joints, the visual analogue scale of patients’ assessment of their general health and the erythrocyte sedimentation rate (ESR) in the first hour. The level of disease activity was interpreted as low (DAS-28≤3.2), moderate (3.2
2.2. Laboratory Investigation
- Seven ml of Peripheral venous blood was withdrawn from each individual and divided into three aliquots; 2 ml of whole blood was collected in EDTA tube for flow cytometry, another 2 ml of whole blood was collected in EDTA tube for ESR determination. The remaining part was collected in serum separator tube, centrifuged at 3500 rpm for 10 min serum was divided into three portions; first one for measurements of RF, second part was used for serum Anti-CCP measurement and Third part of serum was stored at-20°C for CTX-1 detection. Rheumatoid factor (RF) was assayed with a quantitative immunonephelometric method using Minineph Human Rheumatoid factor kit (The Binding Site Group, Code: ZK151.L.R, LOT: 414991-1) and was measured on MININEPH plus instrument. RF was considered positive when the concentration was higher than the cut-off value of the kit (32 IU/ml).Anti-cyclic citrullinated peptide (Anti- CCP) was measured using semi quantitative enzyme-linked immunosorbent assay (ELISA) using QUANTA Lite CCP3 IgG ELISA kit (Inova Diagnostics, REF: 704535, LOT: 049252) with ELISA System AS 1851 Das; Italy (reader) and 16041412Bio Tek; USA (washer) Normal reference level is < 20 Units. Serum CTX-1(Human C-telopeptide of Type I collagen) was assayed using enzyme-linked immunosorbent assay (ELISA) assay (Bioassay Technology Laboratory, Cat. No E1349 Hu, standard curve range: 0.5 ng/ml – 150 ng/ml, sensitivity: 0.21 ng/ml, intra-assay coefficient of variation percent (CV %), < 8% and inter-assay CV %, < 10%). with ELISA System AS 1851 Das; Italy (reader) and 16041412Bio Tek; USA (washer).
2.3. Flow Cytometry for CD19+ CD5+ B Cell Detection
- Samples were prepared using the standard whole blood stain-lyse technique using pre-titrated fluorescein isothiocyanate (FITC) labelled anti-CD19 monoclonal antibodies and phycoerythrin (PE) labelled anti-CD5 monoclonal antibodies, both are supplied by BD Biosciences (USA). Monoclonal antibodies of the same IgG subclass were used as negative controls.10μl of each of the monoclonal antibodies were added to 50μl of the whole blood of each sample and incubated 15 min in the dark at room temperature. 10μl of the isotype control were added to 50μl of the whole blood of each sample and incubated 15 min in the dark at room temperature. 1ml of diluted lysing solution (1 in 10 with distilled water) was added to each tube and incubated 8 min in the dark at room temperature. Tubes were centrifuged at 1200-1500rpm for 5 min. The supernatant was discarded by a non-hesitated single movement. 2 ml of the phosphate buffer saline were added. Tubes were centrifuged at 1200-1500rpm for 5 min. The supernatant was discarded. 200 ml of the phosphate buffer saline were added to the cellular pellet. Stained cells were analyzed on the FACS caliber flow cytometer with the Cell Quest software and 10,000 cells were acquired. Compensation for overlapping signals was set using singly stained samples. Analysis was performed on events falling in the region set around cells of low forward and side scatter.
2.4. Bone Mineral Density Measurement
- BMD was measured for all participants using dual-energy X-ray absorptiometry (DEXA) (Lunar Prodigy; GE Lunar, Madison, WI, United States) at the proximal left femur, lumbar spine (L1–L4), and distal left radius. BMD values were expressed as absolute values (g/cm2) as well as the number of standard deviations (SD) from the mean of healthy, young, sex-matched individuals (the T-score) and the number of standard deviations from the mean of healthy age-matched and sex-matched healthy individuals (the Z-score). Using the WHO classification and the 2005 International Society for Clinical Densitometry (ISCD). A T-score of −2.5 or lower was defined as ‘osteoporosis’; T-score less than −1 but greater than −2.5 was defined as osteopenia; T-score of at least −1 was defined as normal. For BMD measurement in premenopausal women, the ISCD recommendation was used with Zscores ≤-1 SD defined as either “low BMD for chronological age” or “below the expected range for age” and those above −1.0 being “within the expected range for age”. All subjects were tested by the same operator during the study to eliminate operator discrepancies. The average coefficient of variation (CV) for measuring BMD in our device was 1.04%. Quality control procedures were done as per the manufacturer’s recommendations.
2.5. Statistical Analysis
- The recorded data were analyzed using the Statistical Package for Social Sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). The data were presented as number and percentages for the qualitative data; means ± standard deviation and ranges for the quantitative data with parametric distribution and median with inter quartile range (IQR) for the quantitative data with non-parametric distribution using the Kolmogorov–Smirnov test of normality. Student’s t-test were used to compare between the study two groups regarding parametric data and Mann-Whitney test for the non-parametric data while chi-square test was used in the comparison between two groups with qualitative data and Fisher exact test (÷2-test) was used instead of the Chi-square test when the expected count in any cell was less than 5.The comparison between more than two groups with quantitative data and parametric distribution were done by using One Way Analysis of Variance (ANOVA) test. Inter relationships between variables were evaluated using Pearson correlation analysis. Binary logistic regression was used. Multiple linear regression analyses were done, using CD19+CD5+ B lymphocyte as dependent variables. The independent variables were selected if they were correlated with CD19+CD5+ B lymphocyte using Pearson’s correlation coefficient. The confidence interval was set to 95% and the margin of error accepted was set to 5%, so the p-value was considered significant at the level of < 0.05 and P value >0.05 was considered insignificant while P values<0.001 was considered indicate statistically highly significant differences.
3. Results
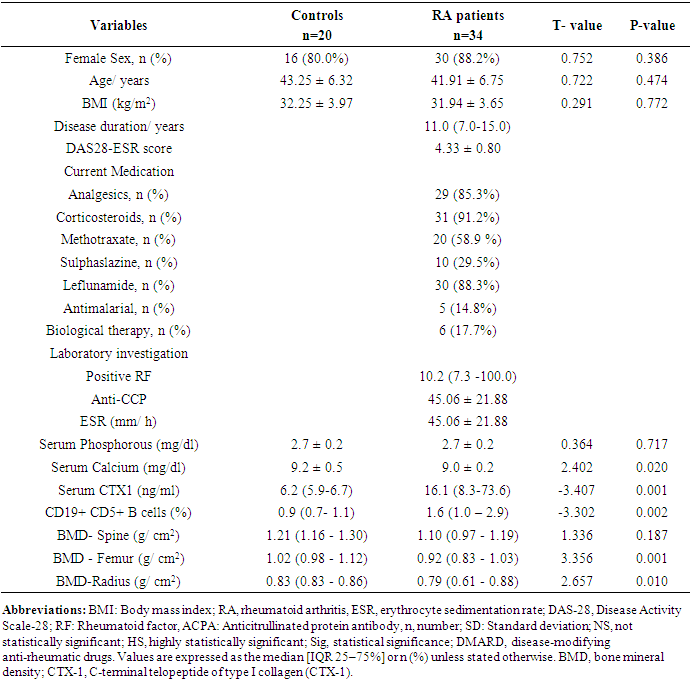
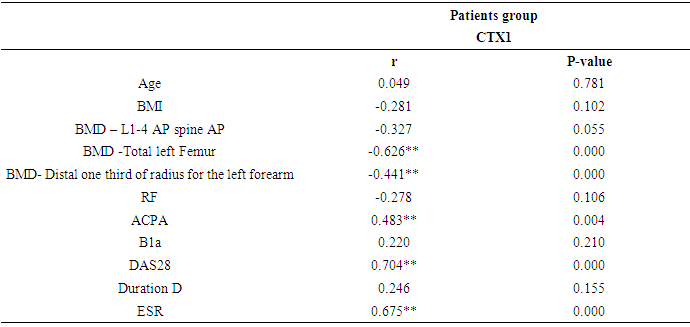
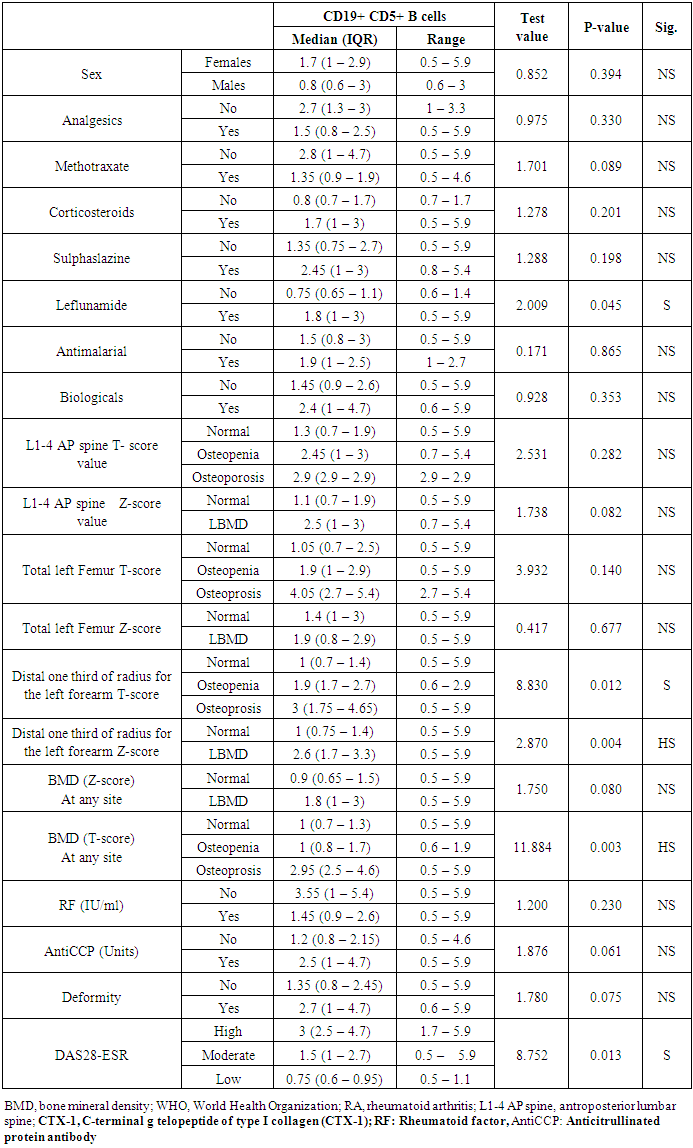
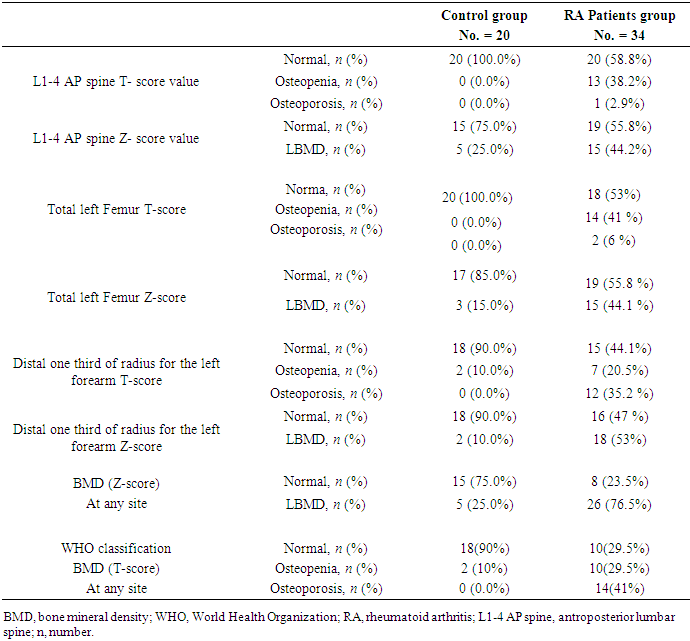
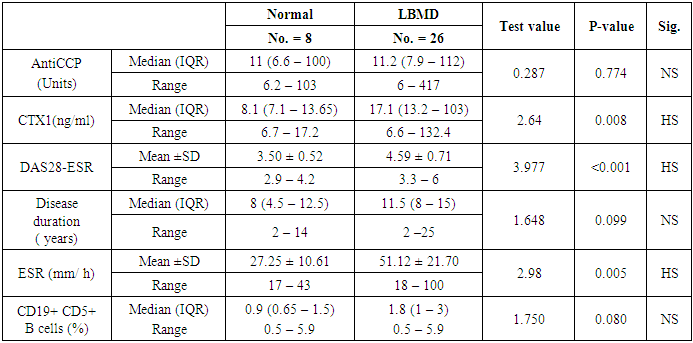
- The present study was enrolled 34 RA patients (mean age 41.91 ± 6.75 years ranged from 29 to 52 years; 88.2% females) and 20 age and sex matched healthy controls (mean age 43.25 ± 6.32 years; 80.0% females) p > 0.05. Demographic, clinical and biochemical characters of the study participants were presented in Table 1. The data were presented as means ± SD, median and interquartile range (IQR) or percent as needed. The median disease duration in RA patients was 11.0 (7.0- 15.0) ranged from 2 to 25 years. Most of RA patients (91.2%) were receiving corticosteroid in a dose of less than 10 mg/day. The median level of RF in RA patients was 10.2 (7.3 -100.0) with about 82.4% seropositive patients however, only 41.2% were positive for Anti-CCP antibodies with mean level of 45.06 ± 21.88. As regard to activity by DAS-28 score, the RA patients were 4 (11.8%) in mild activity, 25 (73.5%) in moderate disease activity, 5 (14.7%) in severe activity and none of them in remission.
|
|
|
|
|
|
|
|
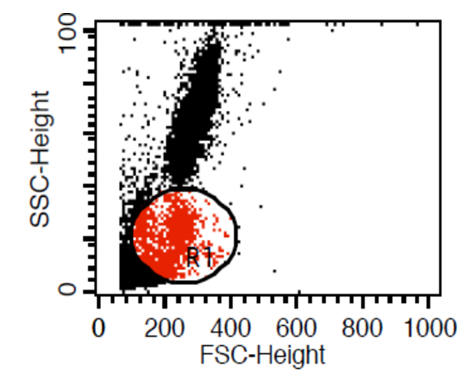 | Figure (1). Gating on lymphocytes using forward side scatter strategy |
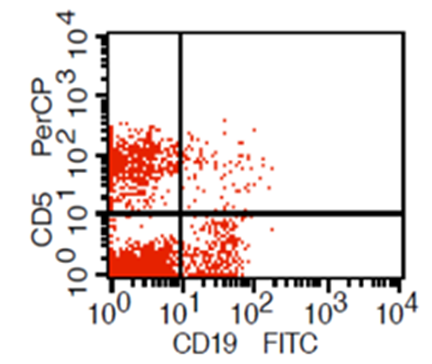 | Figure (2). Expression of CD19 and CD5 on gated lymphocyte (CD19 + cells resemble B lymplocyte,CD19 +CD5+ cells resemble B1a lymophocyte,CD19 – CD5+ cells resemble T lymphocyte) |
4. Discussion
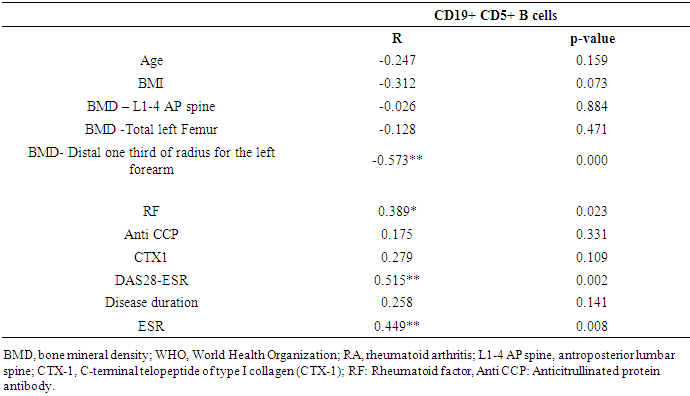
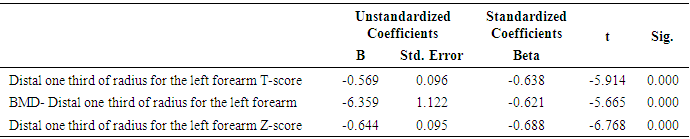
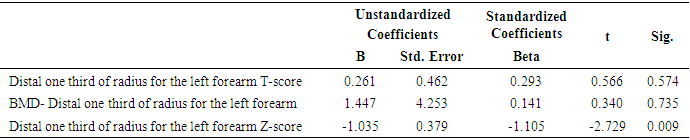
- In this study, we found that RA patients have a significantly higher frequency of CD19+ CD5+ B lymphocytes in comparison to healthy controls. Also, the frequency of CD19+ CD5+ B lymphocytes was significantly increased in high active rheumatoid arthritis group when compared to low active rheumatoid arthritis group indicating that these data can be of diagnostic value and provide information of its involvement in the RA pathogenesis.These results are in agreement with some earlier studies [17-18] which have suggested that the CD5-positive B cell population was increased in the Peripheral blood of patients with RA. One of these studies also reported an increase of CD5-positive B cells in RA synovial fluid, but not to the same level as in peripheral blood [17].Although Liu et al. found no difference in percentages of circulating CD5-positive B cells level between 39 Chinese RA patients with the mean age of 66 years and 41 healthy controls with a mean age of 31 years but they attributed the lack of increase of circulating CD5-positive B cells level in RA patients to the influence of a genetic background [19]. Also, Sowden et al. found no difference in percentages of circulating CD5-positive B cells level between 21 RA patients with the mean age of 66 years and 20 healthy controls with a mean age of 31 years [20].These differences may be further explained by the fact that circulating CD5-positive B cells levels are influenced by many factors including age, sex, menopause, genetic background, disease severity, and disease duration.There is often a significant decrease in circulating CD5-positive B cells levels with advancing age [21].In our study, we have excluded old age and postmenopausal women and hence, we believe that the higher CD5-positive B cell frequencies observed in RA could be due to high disease activity as most of our RA patients had moderate to severe activity scores. Moreover, as the medications received including corticosteroids had no obvious effect on the frequency of CD19+ CD5+ B lymphocytes in our study. This data indicate a link between the frequency of CD5+B lymphocytes and the disease activity.In RA patients, we found that there was a highly significant positive correlation between the frequency of CD19+ CD5+ B lymphocytes and disease activity parameters such as ESR (r = 0,449 and P = 0.008), DAS28-ESR (r = 0.515 and P = 0.002), while, a significant positive correlation with RF (r = 0.389). While, Loza et al. studied 31 RA patients with the mean age of 62.2 ± 2.2 years and reported no correlation between circulating blood levels of CD5+ B lymphocyte levels and disease Activity either in peripheral blood or in synovial fluid in RA patients [22]. This difference between our results and those reported by other investigators might be explained by the difference in the criteria used for diseases activity categorization for RA patients.There was a highly significant negative correlation between the frequency of CD19+ CD5+ B lymphocyte and BMD at forearm (r = -0.573, and P = 0.000) but did not correlate with BMD at lumbar spine, femur or bone resorption marker (CTX-1). In the univariate analysis, the frequency of CD5+ B lymphocyte increased significantly with decreasing BMD measurements at left forearm site (p = 0.000), although this was not found in multivariable analysis after adjustment of DAS28-ESR, RF, and ESR. These findings suggest that CD5+ B lymphocyte is an independent predictor of forearm BMD in RA patients that, by disease activity, largely influences the bone mass, is of weak some importance. The clinical significance of these weak associations remains unclear.Furthermore, up to our knowledge, no other work has discussed the relationship between CD19+ CD5+ B lymphocyte and bone mineral density in RA patients while its relationship to bone resorption marker CXT-1 only one work was found by Engelmannet al. who studied 61 RA patients positive for Anti CCP and reported an association between CD19+ CD5+ B lymphocyte and bone resorption as measured via serum CTX-1 levels in RA patient serum. The variable results could be due to difference in characteristics of the study participants such as age, sex, ethnicity, and influence of treatment and disease activity on bone resorption [23].Osteoporosis is more common in RA patients than in the general population [24]. In our study, BMD-total left femur and BMD-left forearm values were significantly lower in RA patients than controls but not at the lumbar spine site. Kröger et al. [25] as well as Ladder et al. [26] reported lower BMD values in patients with RA. Furthermore, we found that 67.5% of patients with RA (30 premenopausal women and 4 males) had low BMD. The most common site was at the left distal forearm followed by total left femur and L1-4 lumbar spine according to Z-score. Furthermore, we found that 41% of RA patients had osteoporosis in at least one site, 29.5% had osteopenia, and 29.5% had normal BMD values and the most frequent osteoporotic site according to T-score was the left forearm (12 patient, 35%) followed by the total left femur (two patients, 5.5%), and lumbar spine (one patients, 2.9%).This finding runs in accordance with previous published study from Egypt Mehaney et al. studied 40 RA patients (their mean age was 48.9 ± 11.6 years) and reported that osteopenia was found in 37.5% of RA patients and osteoporosis in 35% in at least one site [27]. In the study from India by Shankar et al. of 84 premenopausal women with RA reported osteopenia in 40% of patients at all the three sites and osteoporosis in 22% in at least one site [28].The choice of premenopausal state in woman of this study was selected to exclude the effect of menopause on the occurrence of osteoporosis. The factors contributing to low BMD in our RA patients could be uncontrolled disease as most of them 26 (74.3%) had moderate disease activity, steroid use and low calcium intake or vitamin D deficiency.Additionally, bone loss was mostly detected in peripheral joints suggests that inflammation, cytokines and synovitis mainly cause bone loss in RA patients. Moreover, measurement of left forearm BMD is not routinely recommended in the situation of RA. However, Naumann et al. reported significant correlation between hand BMD and lumbar spine as well as femur BMD in RA patients and concluded that BMD measurement at wrist in addition to spine and femur in RA will assess bone loss properly [29].Serum CTX-1 levels have been shown to be elevated in RA Patients compared to controls which was in agreement with Engelmann et al. [23].and Próchnicka et al. [30] studies. Furthermore, in RA patients it was significantly higher in RA patients with low bone mineral density than those without. Moreover, it was correlated positively with disease activity markers in this study. Próchnicka et al. [30] reported that disease activity was associated with CTX-1 level in RA patients which was in agreement with our study.The strength of our study is that we enrolled a relatively homogeneous group of patients with RA, without any other medical condition that would have caused osteoporosis, and also excluded postmenopausal women with RA which might have contributed to low BMD values. The major limitation is small sample size of patients and controls, besides we could not fully exclude the effects of medication. A larger prospective study powered to take into account these factors may better address this issue.
5. Conclusions
- The frequency of CD19+CD5+ B lymphocyte were higher in Egyptian RA patients compared to normal controls and correlates positively with disease activity parameters and inversely with BMD measurement at forearm site but not at lumbar spine, femur or bone resorption marker (CTX-1) in RA patients suggests a possible contribution of CD19+CD5+B lymphocyte in RA pathogenesis and can be used as diagnosis marker and could thus provide new perspectives for future treatment strategies. However, it's weakly associated with RA related bone loss. For better clarification of the role of CD19+CD5+ B lymphocyte on bone mass in rheumatoid arthritis, larger scale longitudinal studies are recommended. Patients with RA are more susceptible to develop generalized osteoporosis compared with normal population even if they are premenopausal as a result of disease activity so, patients whose disease activity is high despite treatment should be monitored for the risk of osteoporosis.CXT1 as a marker of bone turnover is simple and cost effective that offers an alternative to bone density especially in progressed RA patients as the measurement of bone density is sometimes problematic because of bone deformity and difficulties in positioning the patient while scanning.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML