-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2019; 9(4): 74-78
doi:10.5923/j.cmd.20190904.03

Role of FoxP3+T Regulatory Cells in Chronic HCV and Its Relation to Disease Severity
Khalida ER. El-Refaei1, Doaa SE. Zaky1, Fatma AK. Attia1, Olfat M. Hendy2, Abd-Allah M. Kawzae3
1Department of Internal Medicine, Al-Zahraa Hospital, Al-Azhar University, Cairo, Egypt
2Department of Clinical Pathology, National Liver Institute, El-Menofia University, Egypt
3Departement of Internal Medicine, University Hospital, Tanta University, Egypt
Correspondence to: Doaa SE. Zaky, Department of Internal Medicine, Al-Zahraa Hospital, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: This study was designed to explore the role of Foxp3+ Treg cells in chronic HCV infection by investigating its correlation with HCV load by PCR and its relation to the liver structural injury scored by the grades of activity and stages of fibrosis. Subjects and methods: A prospective cross-sectional observational study was carried out on 50 adult patients with compensated chronic HCV infection and 50 age and sex matched apparently healthy subjects as control. Peripheral blood FoxP3+ Treg cells was detected by three-color flowcytometry. Quantitive HCV PCR was done to determine the viral load. The stages of fibrosis and the grades of activity were determined by per-cutaneous needle liver biopsy. Results: The mean level of Foxp3+ Treg in patients with chronic HCV was significantly higher than the age and sex matched healthy control (136.2 ± 60.8 versus 37.13± 14.76 respectively) P < 0.001. The level of Foxp3+ Treg was significantly higher in patients with high viremia compared to those with moderate viremia and was correlated positively with the level of viremia detected by PCR (r= 0.503 and p value < 0.001). However, no significant difference detected in the level of Foxp3+ Treg in patients with various stages of activity or grades of fibrosis. Conclusion: FoxP3+ Treg cells have essential role in the establishment of chronic HCV infection. Targeting FoxP3+ Treg cells in patients chronically infected with HCV could represent a promising approach to restore functional antiviral immunity and clearance of infection.
Keywords: Foxp3+ Treg, HCV, Viral load
Cite this paper: Khalida ER. El-Refaei, Doaa SE. Zaky, Fatma AK. Attia, Olfat M. Hendy, Abd-Allah M. Kawzae, Role of FoxP3+T Regulatory Cells in Chronic HCV and Its Relation to Disease Severity, Clinical Medicine and Diagnostics, Vol. 9 No. 4, 2019, pp. 74-78. doi: 10.5923/j.cmd.20190904.03.
Article Outline
1. Introduction
- Hepatitis C virus (HCV) infection is a serious health problem that affects more than 170 million people worldwide with high tendency to develop chronic infections [1]. The long-term hepatic impact of HCV infection is highly variable, ranging from minimal change to chronic hepatitis, extensive fibrosis and cirrhosis with increased risk of hepatocellular carcinoma [2]. Failure of an adequate cellular immune response underlies viral persistence in up to 80% of infected individuals [3]. During the acute phase of infection, strong and long-lasting HCV-specific CD4+ and CD8+ T cell responses are associated with viral clearance [4]. Studies with HCV-infected patients have revealed that weak, non-sustained CD4+ T cell response is associated with failure of viral elimination and establishment of chronic infection [5]. HCV specific CD4+ T cells have an altered proliferation rate and altered cytokine production, with a decreased IL-2 secretion [6]. CD4+CD25+Foxp3+ regulatory T (Foxp3+ Treg) cells constitute a specialized T cell population that suppresses the activation, proliferation, differentiation, and effector functions of many types of immune cells, including B cells, NK cells, and dendritic cells [7]. Foxp3+ Treg cells also play an important role in the modulation of antiviral T cell responses and immune-mediated host injury in the acute and chronic phases of viral infection [8]. Foxp3+ Treg cells have been reported to be increased in peripheral blood, and liver infiltrates of chronically HCV infected patients [9]. Moreover, HCV infected hepatocytes are capable of directly inducing development of Foxp3+ Treg cells [10,11]. The high percentage of chronicity in HCV infected patients, as well as the variable long-term hepatic impact of HCV infection make it necessary to know the mechanisms employed by the virus to evade the immune response and to produce hepatic dysfunction. To achieve this goal, this study was designed to explore the role of Foxp3+ Treg cells in chronic HCV infection by investigating its correlation with HCV load by PCR and its relation to the liver structural injury scored by the grades of activity and stages of fibrosis.
2. Subject and Methods
2.1. Study Participants
- This was a prospective cross-sectional observational study. The subjects included were 50 adult patients with compensated chronic HCV infection and 50 age and sex matched healthy controls. The patients were recruited from in-patient department and out-patient clinic of Al-Zahraa University hospital, Cairo, Egypt from august 2016 to September 2017. Informed consents were obtained from all study participants in advance. All procedures were performed in accordance with the guidelines in the Declaration of Helsinki and approved by Research Ethics Committee of Faculty of Medicine for Girls, Al-Azhar University. Exclusion criteria were decompensated liver cirrhosis, other hepatic viral infection, autoimmune diseases, diabetes mellitus or malignancy. All patients were subjected to a standardized questionnaire including: age, sex, possible risk of infection, clinical presentations, comorbidity and medications received. Physical examination was done with special emphasis to resting blood pressure, height and weight. Body mass index (BMI) was calculated as weight/height 2 (kg/m2).
2.2. Laboratory Investigations
- Blood samples were collected from the study participant aseptically. Complete blood picture was performed on Coulter Counter T890 (Coulter Counter, Harpenden, UK). Liver enzymes, including alanine amino-transferase (ALT), aspartate amino-transferase (AST), and alkaline phosphatase (ALP); kidney functions including serum urea and creatinine; fasting blood sugar (FBS) were carried out on Dimension RxL Max analyzer (Siemens Healthcare GmbH - Henkestr. 127, 91052 Erlangen, Germany) by colorimetric techniques. HBsAg and HCVAb were done by using third generation enzyme-linked immunosorbent assay (ELIZA). The viral load was determined by Quantitive HCV PCR.
2.3. Flowcytometry
- Flow cytometric identification of peripheral blood CD4+CD25+FoxP3+ T regulatory cells was done by Becton Dickinson Facs caliber flowcytometry with a standard 3-color filter configuration using PE antihuman FoxP3 staining set (e Bioscience).
2.4. Liver Biopsy
- The patients underwent percutaneous needle liver biopsy with ultrasound guidance. Biopsy sections were prepared and stained. The histopathologic activity was classified as grades from 0 to 4 according to Batts and Ludwig [12]; Grade 0 with no significant inflammation or necrosis, Grade 1 (minimal activity) with portal inflammation and mononuclear cells infiltration confined to the portal areas, Grade 2 (mild activity) with mild portal inflammation, interface hepatitis, and scant lobular spotty necrosis, Grade 3 (moderate activity) with moderate portal inflammation, interface hepatitis, and lobular spotty necrosis, Grade 4 (severe activity) with marked portal inflammation, interface hepatitis, and lobular necrosis, including bridging necrosis.
2.5. Imaging Study
- Fibroscan was done to 42 patients and the stage of fibrosis was determined according to Metavir scoring system [13] as follow; Stage 0 with no fibrosis, stage 1 with fibrous portal expansion, stage 2 with portal expansion and few septa formation, stage 3 with numerous fibrous septa formation, including portal-to-central bridging fibrosis and stage 4 with cirrhosis.
2.6. Statistical Analysis
- Data were collected, revised, coded and analysed by the Statistical Package for Social Science (IBM SPSS) version 20. The qualitative data were presented as number and percentages; the parametric quantitative data were presented as mean± standard deviations while the non-parametric data were presented as median with interquartile range (IQR). The comparison between two groups were done by Chi-square test, independent t-test or Mann-Whitney test as needed. The comparison between more than two independent groups with quantitative data and parametric distribution was done by using one-way analysis of variance (ANOVA) and non-parametric distribution was done by using Kruskall-Wallis test. Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant if P < 0.05.
3. Results
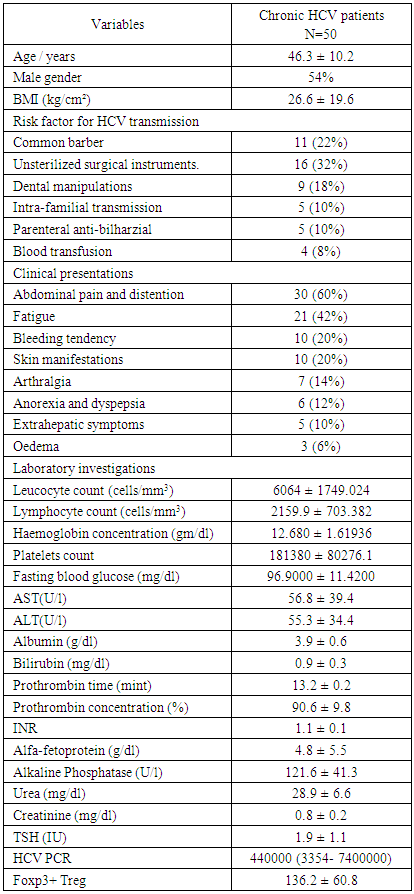
- Clinical and biochemical characteristics of chronic HCV patients (n=50) were presented by table 1. Their mean age was 46.3 ± 10.2 years ranged between 24 and 65 years. Males constituted more than half (54.4%) of the study participant. The most common route of infection in those patients was the unsterilized instrumentation during surgical procedure (16%) followed by Common barber and dental manipulations (11% and 9% respectively). Other routes were less frequent. Abdominal pain and distention were the most common clinical manifestations in the HCV patients of the present study followed by fatigue (21%), bleeding tendency and skin manifestations (10% for each of them). Other manifestations were less frequent. The mean level of Foxp3+ Treg in patients with chronic HCV was significantly higher than the age and sex matched healthy controls (136.2 ± 60.8 versus 37.13± 14.76 respectively) P < 0.001, figure 1.
|
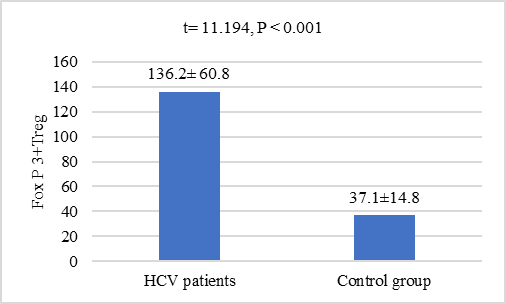 | Figure 1. Comparison of Foxp3+ Treg levels between HCV patients and controls |
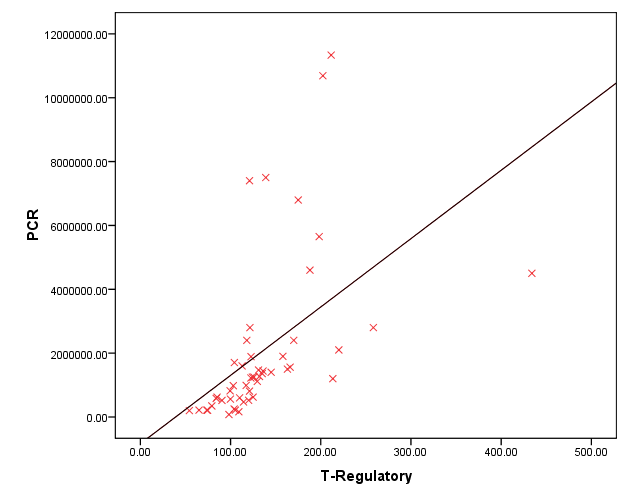 | Figure 2. Correlation between Foxp3+ Treg and HCV PCR |
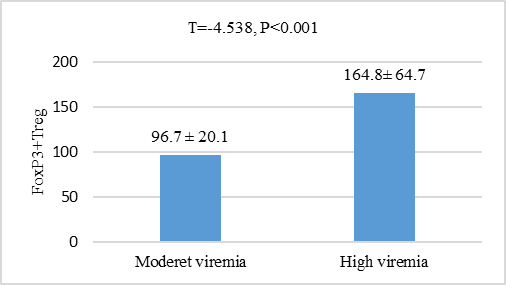 | Figure 3. Comparison of Foxp3+ Treg levels in HCV patients according to degree of viremia based on PCR results |
4. Discussion
- HCV establish chronic persistent infection by escaping the host immune response and can cause immune-mediated liver injury [14]. Foxp3+ Treg cells can modulate viral diseases by suppressing antiviral immune responses and regulating inflammatory host injury [15]. In the present research, we study the role of FoxP3+ Treg cells in patients with chronic HCV and its association with viral load and hepatic structural injury. To achieve this goal, a total of 50 patients with chronic HCV infection were identified, selected and consecutively enrolled in this study as well as 50 age and sex matched healthy controls. The mean value of serum FoxP3+ Treg cells in chronic HCV patient was highly significantly increased when compared to its mean value in the serum of age and sex matched healthy controls (P<0.05). FoxP3+ Treg cells may be contributed to impaired virus-specific T-cell responses and establishing and/or maintaining HCV persistence. In a previous study, Smyk-Pearson et al 2008 [16] compared circulating Treg cells in patients with acute HCV infection who progressed to chronic infection with those in patients who spontaneously resolved the infection. The results demonstrated that chronic progression was associated with long-term maintenance of Treg cells, whereas spontaneous recovery in the acute stage was associated with temporal loss of the suppressive function of Treg cells. Elevated FoxP3+ Tregs in chronic HCV infection was also reported by other studies [14,17-19], however, controversial results demonstrated no difference in the level of Treg cells in chronically HCV infected patients compared to controls [20,21]. The discrepancy between results may be attributed to differences in patient profiles, stage and identification method of circulating Treg cells.The level of Foxp3+ Treg in the present study was also significantly increased in patients with high viremia compared to those with moderate viremia as well as it was positively correlated to viral loade in those patients. HCV may promote the active recruitment of FoxP3+ Treg cells by modulating other immune cells such as dendritic cells and B cells that have ability to generate FoxP3+ Treg cells from naive CD4+ T-cells [18,22]. HCV-infected cells may also directly interact with T-lymphocytes and favor their conversion. The FoxP3+ Treg cells has a potential role to suppress virus-specific CD8+ T-cell proliferation during chronic HCV infection [23,24]. Moreover, FoxP3+ Treg cells were identified within the hepatic lobules in areas of piecemeal and lobular necrosis that enable direct access required to inhibit T cell proliferation [21]. The balance between FoxP3+ Treg cells and effector T cells may determine the level of viremia [25]. However, another study revealed no correlation between the FoxP3+ Treg cells and viral load in chronic HCV infected patients [26]. The different viral activity status related to specific genotypes may be contributed to this contradictory result.The present study failed to correlate the level of FoxP3+ Treg cells to either grades of activity or stage of fibrosis in those patients. Small sample size has been considered as a limitation to this conclusion. The level of Treg cells were not increased with the progression of fibrosis or the grade of inflammations was reported previously [27]. Moreover, another study was reported that the intrahepatic FoxP3+ Treg cells with a fully differentiated and highly activated phenotype were more numerous in those HCV-infected livers showing only limited fibrosis. They suggested that FoxP3+ Treg may play a pivotal role in limiting collateral damage by suppressing excessive HCV-induced immune activation [9]. However, another study was reported substantial correlation between Foxp3(+) CD4(+) Tregs numbers from chronic HCV patients with the stage of fibrosis. Tregs can modulate liver fibrosis by induction of IL-8 and upregulating profibrogenic markers. Hepatic stellate cell activation, but not Treg suppressor function, was blocked by adding a neutralizing IL-8 antibody [28]. Study of the correlation between intera-hepatic FoxP3+ Treg cells and the grade of liver fibrosis and stage of inflammation is still matter of debate and needs further studies.
5. Conclusions
- FoxP3+ Treg cells was elevated in patients with chronic HCV infection and positively correlated to virus load in those patients that indicate its essential role in the establishment of chronic HCV infection. Targeting FoxP3+ Treg cells in patients chronically infected with HCV could represent a promising approach to restore functional antiviral immunity and clearance of infection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML