Nalgaa Abou-Elfattah Tawfik 1, Sarah Younes Abozaid 2, Azza Ali Althoqapy 3, Asmaa Abd Elghany Elsheikh 4, Reem Mohammed Ahmed 5
1Department of Internal Medicine, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
2Department of Clinical Pathology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
3Department of Microbiology and Immunology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
4Department of Community and Occupational Medicine, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
5Department of Biochemistry, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Nalgaa Abou-Elfattah Tawfik , Department of Internal Medicine, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Rheumatoid arthritis (RA) is an autoimmune disease characterized by autoantibodies against citrullinated antigens. Anti-CCP is a test commonly used for the diagnosis of rheumatoid arthritis, anti-mutated citrullinated vimentin (Anti-MCV) is another anti-citrullinated antibody reacting with mutated citrullinated vimentin. There are many studies in comparing anti-MCV and ACCP antibodies for their diagnostic value in rheumatoid arthritis. Anti-MCV especially correlates with higher levels of DAS28 core and joint damage. The aim of this study was to determine the sensitivity and specificity of anti-MCV antibodies in comparison with other inflammatory markers and disease activity in Egyptian rheumatoid arthritis patients. Patients and Methods: Fifty female rheumatoid arthritis patients, were allocated from Rheumatology outpatient clinic and inpatients Internal Medicine Department, Al-Zahraa University Hospital. Their ages ranged from 18 to 74 years with a mean ± SD was (44.6±12.5) years, and fifty healthy volunteers served as a control group. Rheumatoid factor (RF) was measured by latex agglutination, high Sensitive C- Reactive Protein (hs-CRP), Anti-Cyclic Citrullinated Peptides (ACCP) and Anti-Mutated Citrullinated Vimentin (AMCV) were estimated by ELISA. Results: There was statistically highly significant increase in AMCV level with mean ± SD (61.8±47.1 ng/ml) in the patients compared to the control group (8.3±2.9 ng/ml). There was a highly statistically significant correlation between anti-MCV and anti-CCP in the patients (P= 0.001). Also, there was a highly statistically significant correlation between anti-MCV and DAS28 (P= 0.009). The sensitivity of anti-MCV was 92% with 92% specificity, positive predictive value 92%, and negative predictive value 92%. The sensitivity of anti-CCP was 84% with 100% specificity, positive predictive value 100%, and negative predictive value 86.2%. Conclusion: There was a highly significant correlation between anti-MCV and anti-CCP of patients, also highly significant correlation between anti-MCV and DAS28 score. Anti-MCV being more sensitive than anti-CCP while anti-CCP is more specific.
Keywords:
Rheumatoid arthritis, Anti-MCV antibody, Anti-CCP antibody, DAS28 score
Cite this paper: Nalgaa Abou-Elfattah Tawfik , Sarah Younes Abozaid , Azza Ali Althoqapy , Asmaa Abd Elghany Elsheikh , Reem Mohammed Ahmed , Anti-Mutated Citrullinated Vimentin antibodies (Anti-MCV): A Relation with Other Diagnostic Markers in Rheumatoid Arthritis Patients, Clinical Medicine and Diagnostics, Vol. 9 No. 4, 2019, pp. 61-67. doi: 10.5923/j.cmd.20190904.01.
1. Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic inflammation of the synovial joints [1], that often leads to the destruction of bone and cartilage [2]. Women were affected two to three times more commonly than men [3]. The cause of rheumatoid arthritis is not clear; it is believed to involve a combination of genetic and environmental factors [4]. The rheumatoid factor, used as a diagnostic marker for RA, is nonspecific and may be present in healthy individuals or those with other autoimmune diseases [1]. Anti-CCP is a specific prognostic marker for RA, which predict progression of the disease [5]. Antibodies against mutated citrullinated vimentin (anti-MCV) have been evaluated for the diagnosis of RA, showing high sensitivity and specificity for the diagnosis of the disease [6]. Vimentin is an intermediate filament that is widely expressed by mesenchymal cells and macrophages and easy to detect in the synovium [5]. Citrullinated peptide activates T-lymphocytes by binding on HLA-DR4 on the surface of antigen-presenting cells and contribute to the pathogenesis of rheumatoid arthritis [7]. Anti-MCV also correlates with higher levels of DAS28 and joint damage [8]. This study aimed to determine the sensitivity and specificity of anti-MCV antibodies in comparison with other inflammatory markers and disease activity in Egyptian patients.
2. Subjects and Methods
2.1. Study Design
This study is a case-control study conducted on a hundred participants, they were divided into two groups: Group I: Fifty rheumatoid arthritis female patients were allocated to the study from Rheumatology outpatients clinic and inpatients Internal Medicine Department, Al-Zahraa University Hospital, in the period between March 2018 to December 2018, after written consent from all patients and control subjects according to the ethical committee of the university. Patients were diagnosed according to the 2010 ACR/EULAR RA classification criteria. They were subdivided according to disease activity into three subgroups: Group Ia: Patients with mild disease activity: included 15 patients whose DAS28 score <3.2. Group Ib: Patients with moderate disease activity: included 17 patients whose DAS28 score ≥3.2 and <5.1. Group Ic: Patients with severe disease activity: Included 18 patients whose DAS28 score ≥5.1. Group II: Fifty healthy volunteers served as a control group. Inclusion criteria: Adult female rheumatoid arthritis patients. Exclusion criteria: Other autoimmune diseases such as systemic lupus erythematosus, overlapping syndrome, myasthenia gravis, Hashimoto thyroiditis, pregnant or nursing patients.
2.2. Blood Sampling and Measurements
All patients and control subjects were subjected to the following laboratory investigations: Blood samples were obtained by venipuncture technique 2 ml of whole blood on EDTA tube for determination of complete blood count (CBC) by Sysmex KX-21N (Kobe, Japan) an automated hematology cell counter and erythrocyte sedimentation rate (ESR) determination by Westergren method, another 3 ml of blood in serum separator tube, for routine chemistry analysis, after centrifugal separation of serum total cholesterol (TC), triglyceride (TG), serum calcium and phosphorus were done on Cobas C311 (Germany) by using kits of Roche (Germany) at the same day. The rest of serum samples were stored at -20°C for later analysis of Rheumatoid factor (RF), High Sensitive C- Reactive Protein (hs-CRP), Anti-Cyclic Citrullinated Peptides (ACCP) and Anti-Mutated Citrullinated Vimentin (AMCV). Rheumatoid factor (RF) was measured by semi-quantitative latex agglutination kit supplied from Omega Diagnostics, Scotland, United Kingdom, A vitex RF (Ref OD063), negative results are at an RF concentration below 8 IU/L, and positive results are at an RF concentration above 8 IU/L. High Sensitive C- Reactive Protein (hs-CRP) was measured by quantitative solid-phase enzyme-linked immunosorbent assay (ELISA) kit supplied from BioScience, San Francisco, USA, Cat. No. 10603 (Sensitivity: normal expected values in adult serum: 68-8200 ng/ml); using AS 1851 Das; Italy (reader) and 16041412 Bio Tek; USA (washer). Anti-Cyclic Citrullinated Peptide 3 (ACCP3) was measured by semi-quantitative ELISA for the detection of IgG anti-CCP3antibodies in patient sera (using QUANTA Lite CCP3 IgG ELIZA kit from Inova Diagnostics, San Diego, USA) (Ref. No. 704535); using AS 1851 Das; Italy (reader) and 16041412 Bio Tek; USA (washer). Negative results are at a concentration below 20 U/mL; positive results are at a concentration above 20 U/mL (weak positive 20-39, moderate positive 40-59, strong positive equal or above 60 U/mL). Anti-Mutated Citrullinated Vimentin (AMCV) was measured by quantitative double-antibody sandwich ELISA kit supplied from Bioassay Technology Laboratory, China, Cat. No. E0474 Hu (Standard curve range: 0.5 ng/ml - 200 ng/ml and sensitivity: 0.25 ng/ml); using AS 1851 Das; Italy (reader) and 16041412 Bio Tek; USA (washer).
2.3. Statistical Methods
Data were collected, revised, coded, and entered to the Statistical Package for Social Science (IBM Corp. Released in 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp. The sample size was calculated according to the following formula [9]: 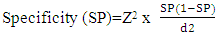 SP= Specificityz = Confidence Interval normal distribution value i.e. for 95%, z = 1.96d = prevalence of the disease in the test populationSpecificity considered 96.6% [10]Prevalence of RA in population= 1% worldwide [11]The normality tests were done. The quantitative data were presented as mean, standard deviations when their distribution found parametric, and median, interquartile range (IQR) for non-parametric data. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test and Mann Whitney U test for non-parametric one. Comparison of quantitative variables between more than two groups was done using ANOVA for parametric data and the Kruskal Wallis test for nonparametric one. Correlation between various variables was done using Pearson and Spearman rank correlation equation for normal and non-normal distributed variables, respectively. P-values less than 0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS version 23.
SP= Specificityz = Confidence Interval normal distribution value i.e. for 95%, z = 1.96d = prevalence of the disease in the test populationSpecificity considered 96.6% [10]Prevalence of RA in population= 1% worldwide [11]The normality tests were done. The quantitative data were presented as mean, standard deviations when their distribution found parametric, and median, interquartile range (IQR) for non-parametric data. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test and Mann Whitney U test for non-parametric one. Comparison of quantitative variables between more than two groups was done using ANOVA for parametric data and the Kruskal Wallis test for nonparametric one. Correlation between various variables was done using Pearson and Spearman rank correlation equation for normal and non-normal distributed variables, respectively. P-values less than 0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS version 23.
3. Results
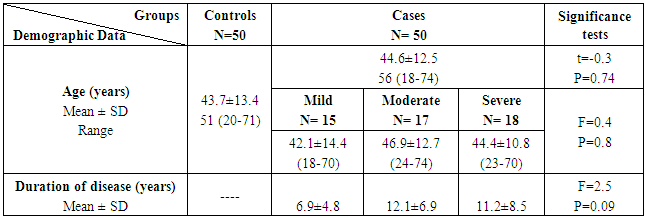
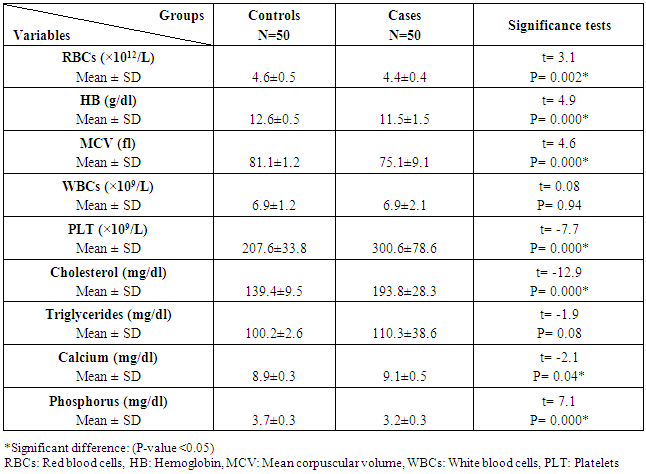
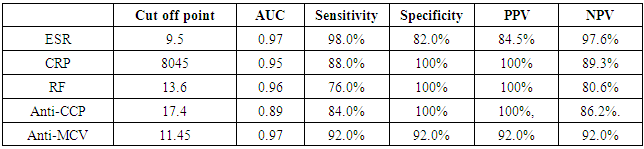
The study included two groups: patients' group (fifty RA female patients, (100%), compared to the control group (50 healthy female subjects). Table (1) shows the demographic data of patients included in the study; their age ranged from 18-74 years, with mean ± SD (44.6±12.5) years and control group their age ranged from 20-71 years, with mean ± SD (43.7±13.4). Clinical and laboratory characteristics of the RA patients and controls are demonstrated in (Tables 2 and 3). There were highly statistically significant differences between cases and controls as regards ESR, CRP, ACCP, RF, and AMCV. AMCV level mean ± SD (61.8±47.1 ng/ml) in cases while in control group mean ± SD (8.3±2.9 ng/ml) as shown in table (3) and figure (1). Comparison between the subgroups of cases (mild, moderate, and severe) there was a highly significant difference as regard ESR, ACCP, AMCV, DAS28 score. Anti-MCV levels in the cases were detected with a mean of (41.5±29 ng/ml), (49.8±32.7 ng/ml), and (90.1±57.8 ng/ml) (P=0.004) for mild, moderate, and severe groups of cases respectively. Anti-CCP levels showed a high significant difference between cases subgroups (mild, moderate and severe) with a mean ± SD (34.9±30.4 ng/ml), (187.2±177.3 ng/ml), and (285.2±149.1 ng/ml) (P=0.000) for mild, moderate and severe cases respectively (Table 4). The cases were divided according to anti-MCV into 2 groups; anti-MCV positive and anti-MCV negative. Both groups were compared as regards some characteristics. The anti-CCP titers were significantly higher in RA patients with a positive anti-MCV compared to those with a negative anti-MCV (P= 0.002). There were no significant differences between anti-MCV positive and negative patients as regards to DAS28 score, ESR, RF, CRP, and disease duration (Table 5). There was a highly significant correlation between anti-MCV and anti-CCP among patients (P = 0.001), also a highly statistically significant correlation between anti-MCV and DAS28 score (P = 0.009) (Table 6 and Figures 2 & 3). The sensitivity of anti-MCV was 92% with 92% specificity, positive predictive value 92%, and negative predictive value 92% (Figure 4). The sensitivity of anti-CCP was 84% with 100% specificity, positive predictive value 100%, and negative predictive value 86.2% (Figure 5). Our results revealed anti-MVC being more sensitive than anti-CCP while anti-CCP is more specific (Table 7 and Figures 4 & 5). Table 1. Demographic data of the rheumatoid arthritis patients and control
 |
| |
|
Table 2. Laboratory Findings of the rheumatoid arthritis patients and control
 |
| |
|
Table 3. Inflammatory markers of the rheumatoid arthritis patients and control
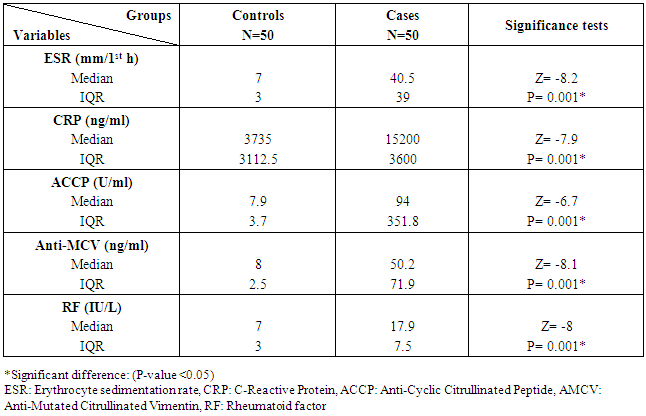 |
| |
|
Table 4. Inflammatory markers of the rheumatoid arthritis patients
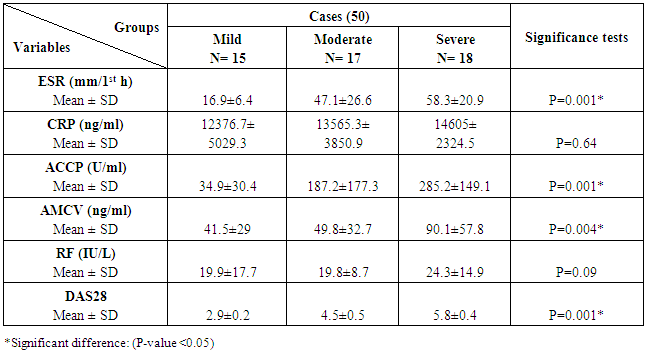 |
| |
|
Table 5. Anti-Mutated Citrullinated Vimentin (AMCV) positive and negative (according to cut off point by ROC curve) in rheumatoid arthritis (RA) patients
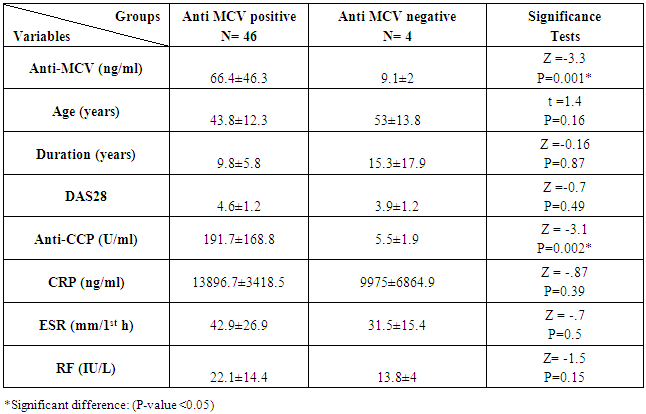 |
| |
|
Table 6. Correlation between Anti-MCV and other inflammatory markers in rheumatoid arthritis patients
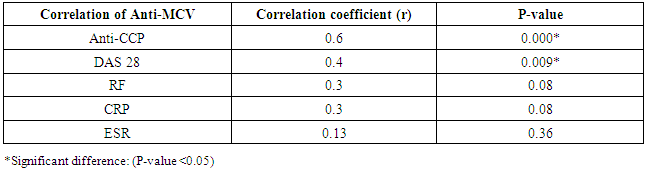 |
| |
|
Table 7. Diagnostic accuracy of ESR, CRP, RF, Anti-CCP, and Anti-MCV in the diagnosis of rheumatoid arthritis
 |
| |
|
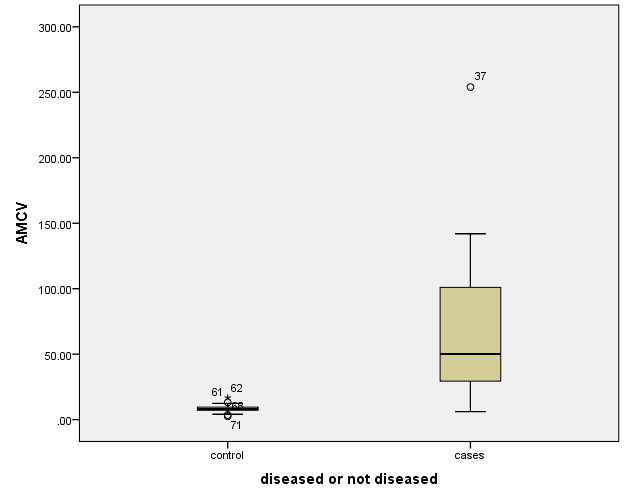 | Figure 1. Anti-MCV level in rheumatoid arthritis patients and controls |
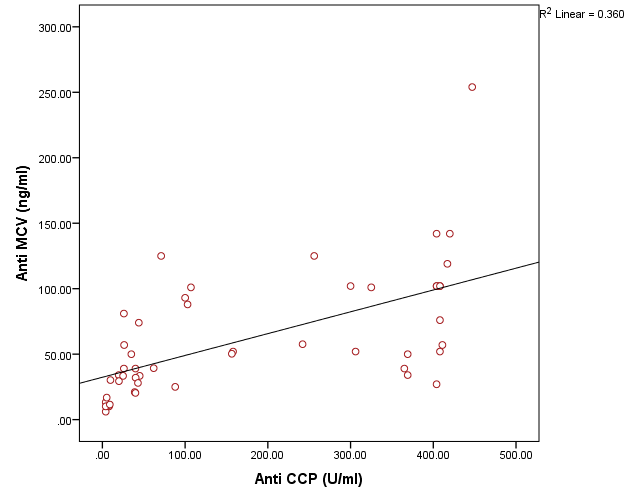 | Figure 2. Correlation between AMCV and ACCP in rheumatoid arthritis patients |
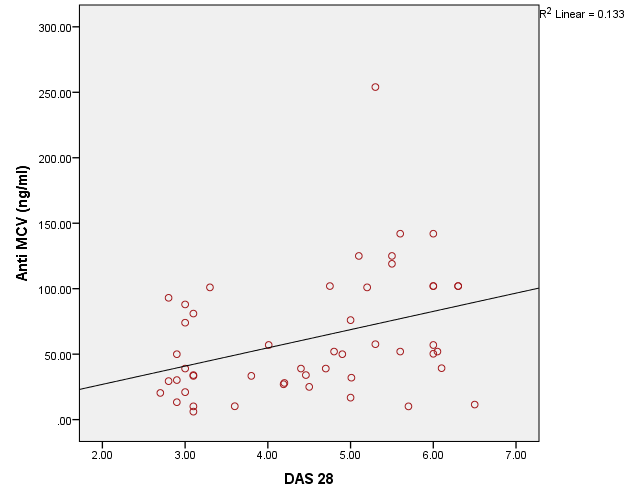 | Figure 3. Correlation between AMCV and DAS 28 score in rheumatoid arthritis patients |
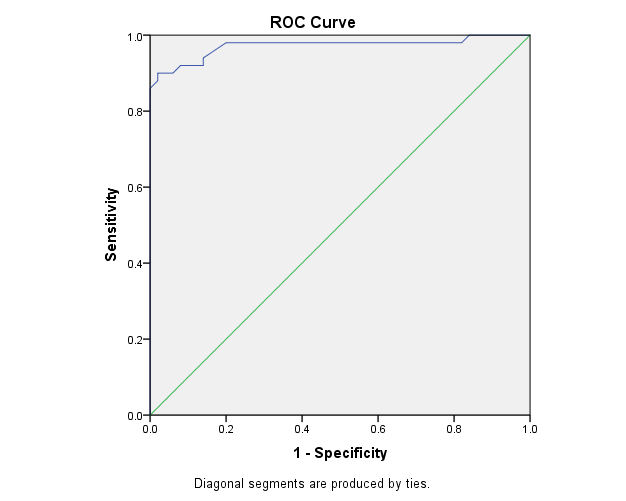 | Figure 4. The ROC curve analysis of Anti-MCV in demonstration of rheumatoid arthritis |
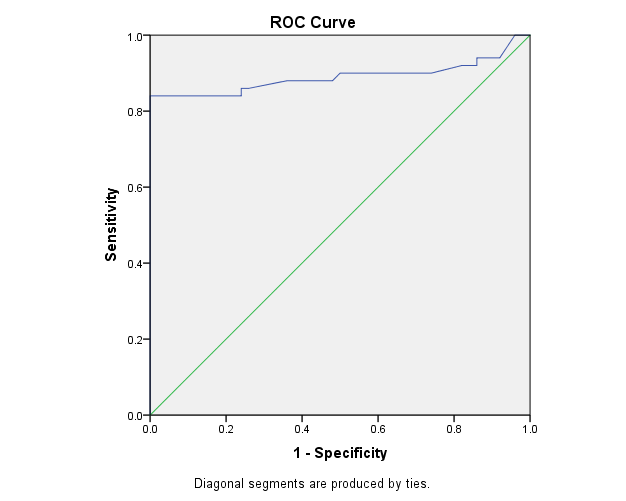 | Figure 5. The ROC curve analysis of Anti-CCP in demonstration of rheumatoid arthritis |
4. Discussion
Rheumatoid arthritis is the most common inflammatory joint disease [5]. Numerous serological markers of RA have been described [7]. Rheumatoid factor and anti-cyclic citrullinated antibodies (anti-CCP) are present prior to the appearance of clinical symptoms of arthritis [12]. The diagnosis of RA has been improved by the detection of auto-antibodies against different citrullinated antigens as anti-mutated citrullinated vimentin antibodies (Anti-MCV) [13].In the presented study there was statistically highly significant increase between cases and controls as regard ESR, CRP, ACCP, RF, and AMCV, these results agreed with Osman et al. [5] and Abou El-Fetou and Abozaid [14]. Also, our result revealed highly significant increase between groups of cases: mild, moderate, and severe as regards ESR, ACCP, AMCV, and DAS28 score.In the presented study there is a significant increase was found between anti-MCVpositive and anti-MCV negative groups as regards ACCP (p=0.002), this result agreed with El Shazly et al. [7] while there were no significant differences found between anti-MCV positive and anti-MCV negative groups as regards of the Disease Activity Score28 (DAS28), ESR, RF, CRP and duration of disease [7]. A significant positive correlation was present between anti-MCV and anti-CCP in patient with rheumatoid arthritis, this agreed with Osman et al. [5] and El Shazly et al. [7], also, it was in agreement with Khalifa et al. [15]. This is explained by the cross-reactivity between anti-MCV, and anti-CCP antibodies indicate that anti-MCV and anti-CCP target some shared epitopes, which may explain the high positive correlation between these antibodies [16]. Also, a positive correlation was present between anti-MCV and DAS28 score; this result is in agreement with Osman et al. [5]. In the present study, the non-significant correlation was found between RF and anti-MCV, this result agrees with Osman et al. [5] and disagrees with Al-Shukaili et al. [2] who reported a significant positive correlation between Anti-MCV and RF.We found that the sensitivity of Anti -MCV in the diagnosis of RA was (92.0%), and specificity was (92.0%), this agreed with the study of El Shazly et al. [7] as they found that the sensitivity of anti-MCV is 84% and the specificity is 80%, also agreed with Poulsom and Charles [4] who reported a sensitivity of 84% and specificity of 87%, also Bang et al. [17], found that sensitivity of Anti MCV was 82% and specificity 88%, respectively, while Liu et al. [18], reported a lower sensitivity and higher specificity (78.2% and 93.4%), respectively, Sizova [19] reported a lower sensitivity (53.3%) and comparable specificity (83.3%). Also, in the present study, the Sensitivity of Anti-CCP in diagnosing RA is (84.0%), and specificity is (100.0%), this result agrees with the studies of El Shazly et al. [7], they found that sensitivity for anti-CCP was 70% and specificity was 100%. We found that anti-MCV antibodies have the highest sensitivity, and anti-CCP antibodies have the highest specificity.
5. Conclusions
The diagnostic performance of anti-MCV is comparable to anti-CCP in RA, and the lower diagnostic accuracy of anti-MCV was mainly due to its relatively lower specificity. Also, anti-MCV correlates with DAS28 score.
References
| [1] | Lee YH, Bae SC and Song GG (2015). Diagnostic accuracy of anti-MCV and anti-CCP antibodies in rheumatoid arthritis: A meta-analysis. Z Rheumatol, 74(10): 911-918. |
| [2] | Al-Shukaili A, Al-Ghafri S, Al-Marhoobi S and Alkaabi J (2012). Evaluation of anti-mutated citrullinated vimentin antibodies, anti-cyclic citrullinated Peptide antibodies and rheumatoid factor in Omani patients with rheumatoid arthritis. Int J Rheumatol, 2012: 285854. |
| [3] | Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD and Tanasescu R (2010). Extra-articular Manifestations in Rheumatoid Arthritis. Maedica (Buchar), 5(4): 286-291. |
| [4] | Poulsom H and Charles PJ (2008). Antibodies to citrullinated vimentin are a specific and sensitive marker for the diagnosis of rheumatoid arthritis. Clin Rev Allergy Immunol, 34(1): 4-10. |
| [5] | Osman KS, Aly LH, Saedii AA, Abbas HT and Sadek HA (2014). Anti-Mutated Citrullinated Vimentin (Anti-MCV) Antibodies as a Diagnostic Aid for Rheumatoid Arthritis. J Clin Cell Immunol, 5: 263. |
| [6] | Gonzalez-Lopez L, Rocha-Muñoz AD, Ponce-Guarneros M, Flores-Chavez A, Salazar-Paramo M, Nava A, et al. (2014). Anti-cyclic citrullinated peptide (anti-CCP) and anti-mutated citrullinated vimentin (anti-MCV) relation with extra-articular manifestations in rheumatoid arthritis. J Immunol Res, 2014: 536050. |
| [7] | El Shazly RI, Hussein SA, Raslan HZ and Elgogary AA (2014). Anti-mutated citrullinated vimentin antibodies in rheumatoid arthritis patients: Relation to disease activity and manifestations. Egyptian Rheumatologist, 36(2): 65-70. |
| [8] | Yousefghahari B, Alhooei S, Soleimani-Amiri MJ and Guran A (2013). Comparison of sensitivity and specificity of anti-CCP and anti-MCV antibodies in an Iranian cohort of patients with rheumatoid arthritis. Caspian J Intern Med, 4(3):702-706. |
| [9] | Hajian-Tilaki K (2014). Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform, 48: 193-204. |
| [10] | Mansour HE, Metwaly KM, Hassan IA, Elshamy HA and Elbeblawy MM (2010). Antibodies to mutated citrullinated vimentin in rheumatoid arthritis: diagnostic value, association with radiological damage and axial skeleton affection. Clin Med Insights Arthritis Musculoskelet Disord, 3: 33-42. |
| [11] | Gibofsky A (2012). Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care, 18(13 Suppl): S295-302. |
| [12] | van Venrooij WJ, van Beers JJ and Pruijn GJ (2011). Anti-CCP antibodies: the past, the present and the future. Nat Rev Rheumatol, 7(7): 391-398. |
| [13] | Luime JJ, Colin EM, Hazes JM and Lubberts E (2010). Does anti-mutated citrullinated vimentin have additional value as a serological marker in the diagnostic and prognostic investigation of patients with rheumatoid arthritis? A systematic review. Ann Rheum Dis, 69(2): 337-344. |
| [14] | Abou El-Fetou S and Abozaid HS (2013). Diagnostic value of antibodies against a modified citrullinated vimentin in rheumatoid arthritis. Open Journal of Rheumatology and Autoimmune Diseases, 3(4): 185-191. |
| [15] | Khalifa IA, Lotfy AM, Elsayed MA and Abd-Elfattah A (2013). Anti-mutated citrullinated vimentin antibodies in rheumatoid arthritis compared with anti-cyclic citrullinated peptides. J Am Sci, 9: 160-166. |
| [16] | Engelmann R, Brandt J, Eggert M, Karberg K, Krause A, Neeck G and Mueller-Hilke B (2009). The anti-mutated citrullinated vimentin response classifies patients with rheumatoid arthritis into broad and narrow responders. J Rheumatol, 36(12): 2670-2674. |
| [17] | Bang H, Egerer K, Gauliard A, Lüthke K, Rudolph PE, Fredenhagen G, et al. (2007). Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum, 56(8): 2503-2511. |
| [18] | Liu X, Jia R, Zhao J and Li Z (2009). The role of anti-mutated citrullinated vimentin antibodies in the diagnosis of early rheumatoid arthritis. J Rheumatol, 36(6): 1136-1142. |
| [19] | Sizova L (2012). Diagnostic value of antibodies to modified citrullinated vimentin in early rheumatoid arthritis. Hum Immunol, 73(4): 389-392. |


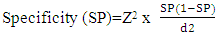 SP= Specificityz = Confidence Interval normal distribution value i.e. for 95%, z = 1.96d = prevalence of the disease in the test populationSpecificity considered 96.6% [10]Prevalence of RA in population= 1% worldwide [11]The normality tests were done. The quantitative data were presented as mean, standard deviations when their distribution found parametric, and median, interquartile range (IQR) for non-parametric data. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test and Mann Whitney U test for non-parametric one. Comparison of quantitative variables between more than two groups was done using ANOVA for parametric data and the Kruskal Wallis test for nonparametric one. Correlation between various variables was done using Pearson and Spearman rank correlation equation for normal and non-normal distributed variables, respectively. P-values less than 0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS version 23.
SP= Specificityz = Confidence Interval normal distribution value i.e. for 95%, z = 1.96d = prevalence of the disease in the test populationSpecificity considered 96.6% [10]Prevalence of RA in population= 1% worldwide [11]The normality tests were done. The quantitative data were presented as mean, standard deviations when their distribution found parametric, and median, interquartile range (IQR) for non-parametric data. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test and Mann Whitney U test for non-parametric one. Comparison of quantitative variables between more than two groups was done using ANOVA for parametric data and the Kruskal Wallis test for nonparametric one. Correlation between various variables was done using Pearson and Spearman rank correlation equation for normal and non-normal distributed variables, respectively. P-values less than 0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS version 23.




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





