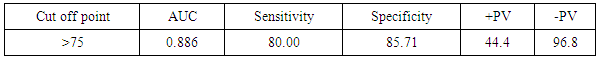-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2019; 9(3): 55-60
doi:10.5923/j.cmd.20190903.03

Egyptian Study of Post-transplant sCD30 as a Predictor of Kidney Graft Outcome
Ragaa Ramadan Mohamed 1, Ahmed Hassan Elthakaby 2, Amal Hussein Ibrahim 1, Hala Ibrahim Mohamed 3, Azza A. I. El Menyway 3, Aisha Mohamed Ahmed Elshimy 3, Safa Arafat Abdel-Moneim 4
1Department of Internal Medicine and Nephrology, Faculty of Medicine for Girls – Azhar University, Cairo, Egypt
2Department of Nephrology, National Institute of Nephrology, Cairo, Egypt
3Department of Clinical Pathology, National Institute of Nephrology, Cairo, Egypt
4Department of Nephrology, Dar Elsalam Hospital, Ministry of Health, Cairo, Egypt
Correspondence to: Amal Hussein Ibrahim , Department of Internal Medicine and Nephrology, Faculty of Medicine for Girls – Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

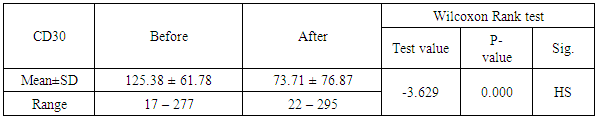
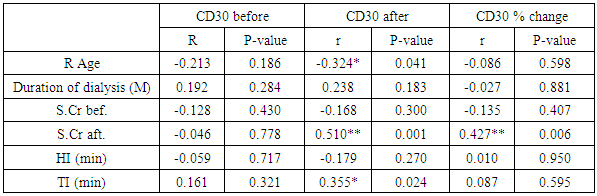
Background: Previous studies revealed the potential value of the soluble CD30 level (sCD30) a marker for T helper 2-type cytokine-producing Tcells as biomarker for the risk of acute rejection and graft failure after renal transplantation. Aim of the Work: We examined the predictive value of pre-transplant and post-transplant sCD30 levels for kidney transplantation outcome. Methods: This is an observational study of renal transplant patients receiving triple immunosuppressive therapy, we examined whether sCD30 can be used as a predictor of renal graft rejection. 40 patients were participating in our study, 32 males and 8 females aged 17 to 62 years old. We examined the predictive value of pre-transplant and post-transplant sCD30 levels for kidney transplantation outcome. SCD30 was measured by ELISA in serum taken day 0 before and day 30 after transplantation. Results: Our study revealed that Serum levels of sCD30 decreased significantly in samples post-transplantation compared with pre-transplantation the range of CD30 level day 0 before transplantation which was 17-277 ngm/ ml, there was significant positive relation between level of CD30 after transplantation and the early outcome of the patients, also there was significant inverse relation between level Cd30 after transplantation and the age of recipients P-value< 0.041 and finaly reverse realtion between Cd30 after transplantation and the transient ischemia during operation (P-value 0.024). Conclusions: Our results did not suggest that the measurement of sCD30 may be used as a valuable biomarker in renal transplantation. Increased levels may be related to a decrease in its renal elimination.
Keywords: Soluble CD30 levels pre and post transplantation, Outcome of renal transplantation, Kidney rejection
Cite this paper: Ragaa Ramadan Mohamed , Ahmed Hassan Elthakaby , Amal Hussein Ibrahim , Hala Ibrahim Mohamed , Azza A. I. El Menyway , Aisha Mohamed Ahmed Elshimy , Safa Arafat Abdel-Moneim , Egyptian Study of Post-transplant sCD30 as a Predictor of Kidney Graft Outcome, Clinical Medicine and Diagnostics, Vol. 9 No. 3, 2019, pp. 55-60. doi: 10.5923/j.cmd.20190903.03.
1. Introduction
- Early recognition of acute rejections (AR) may help in treatment and avoidance of long term sequelae currently, panel reactive antibodies (PRA) are generally accepted as the indicators of immunological status of renal graft candidates and the predictors of renal graft outcome. [1] Recently serum levels of sCD30 have received much attention. [2,3] Several reports show that elevated sCD30 levels, pre and post transplantation are associated with a poor prognosis for long term kidney graft survival. [4,5] These studies found higher sCD30 levels in allograft recipients and a good predictor of impending AR. The soluble CD30 molecule, a member that belongs to the tumor necrosis factor and nerve growth factor receptor superfamily. [6] It is expressed on CD4+ and CD8+ activated T cells that secrete Th2-type cytokines superfamily, is a 120 kDa cell-surface glycoprotein that is expressed on the cell surface of activated CD4_ Th2 and CD8_ T cells, as well as in other lymphoma cells. It is believed that CD30 has immunoregulatory functions, especially in alloimmune responses. Once activated CD30 is secreted from the cell surface and so can be measured in the circulation in its soluble form (sCD30). [6] soluble CD30 could be found in healthy humans but in little amounts, in the other hand increased level of sCD30 indicates certain pathophysiological situations such as systemic lupus erythematosus, rheumatoid arthritis, certain viral infections and adult T cell leukemia/lymphoma. [7]Many studies have shown that pretransplantation serum levels of sCD30 maybe considered as a prognostic biomarker for outcome in kidney transplantation, independent of alloantibody sensitization. [1-7] It has been beleived that both HLA antibodies and high sCD30 may considered as indicators of poor graft outcome. [2-8] However, serum levels of sCD30 is affected by individual; variations and time dependent changes. [9]The aim of our study is to analyze the predictive value of pre-transplant and post-transplant sCD30 levels for kidney transplantation outcome in a single-center patient’s cohort study.
2. Patients and Methods
- This is prospective observational single center study on a possible role of sCD30 during the pre-transplant and post-transplant phase. We conducted on 40 patients received living kidney transplantation at the National Institute of Urology and Nephrology in Cairo after taking a written consent. From January 2017 to July 2018, 40 consecutive patients received a kidney allograft at National institute of Nephrology and Urology (NINU). The criteria of inclusion were: the adult recipient of kidney allograft from related or unrelated donor, male or female, negative cross-match, and available follow-up data. Certain patients were excluded with diseases that elevate serum CD30 as multipule scelerosis, rheumatoid arthritis, systemic lupus erythematosus, atopic dermatitis. The final candidates, who matched the inclusion criteria, consisted of 40 patients, 32 males and 8 females aged 17 to 62 years old. Token samples were investigated for level of sCD30 pre-transplantation and the day 30 post transplantation. ALL patients will be subjected to Full history including dialysis history & clinical examination with stress on etiology of primary kidney disease and associated complications. Full review of medications the patients received including immunosuppression. Full review of routine work up of transplantation preparation.Pretransplantion evaluation: All patients were subjected to Blood group done, HLA typing and HLA mismatch, PRA, Virology (HCV, HBV, HIV, CMV), Routine workup laboratory pretransplant preparations: KFTs, CBC, Liver enzymes, immunological markers.Immunotherapy: All recipients received triple maintenance immunosuppressive therapy consisting of calcineurin-inhibitor (cyclosporine A or tacrolimus), antiproliferative agent (mycophenolate mofetil (MMF)), and steroid. And drug levels (C0, Fk level) was done.All patients received classical induction therapy before transplantation in the forum of pulse steroid, calcineurin- inhibitor, MMF and thirty percent of patients received anti-CD25 antibodies Basiliximab (Simulect®) or Anti-thymocyte globulin (ATG) for induction.(patients with HLA mismatch score more than 3, and elevated PRA percentage). Serum sCD30 was measured from the collected samples of 40 kidney allograft recipients transplanted at our center. Samples were collected at day 0 pre-transplantation and at day 30 post transplantation the sera of patients were collected and stored at -20°C. Statistical AnalysisNormally distributed parametric data are expressed as mean ± SD; Pearson’s correlation coefficient (r) was calculated. Continuous nonparametric data are expressed as median (interquartile range); for comparison we used the Kruskal-Wallis test with post-hoc-comparisons, Mann-Whitney’s or Wilcoxon’s tests. P value = level of significance: P-value > 0.05: Non-significant. P-value < 0.05: Significant. P-value < 0.01: Highly significant.
3. Result
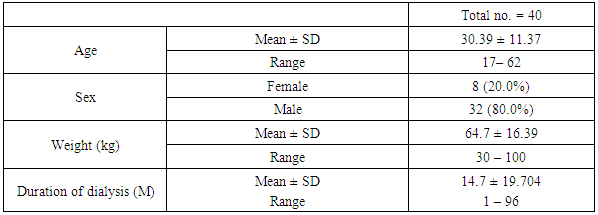
- In our study The range of age for all patients was from 17 – 62 years, with mean±SD30.39±11.37 years, the male gender number was higher than female (80% for male and 20% for female). 97.5% of patients was nonsmoker.The range of duration of dialysis per months was ranging from 1 month to 96 months with mean±SD value 14.7±19.704 months Table 1.
|
|
|
|
|
4. Discussion
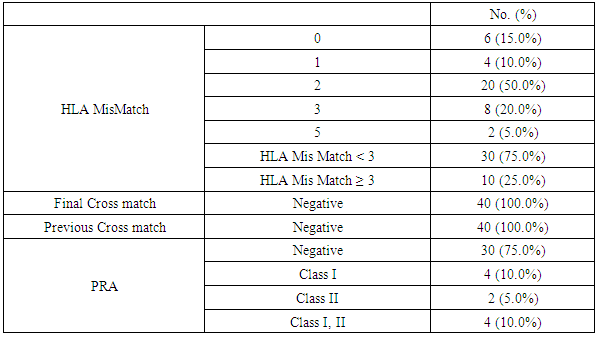
- One of the most important issues in kidney transplant is immunologic tolerance of the transplanted organ by the recipient. Immunologic status of patients can have a direct effect on transplant outcome, and presence of preformed anti-human leukocyte antigen (HLA) antibodies may lead to decreased graft survival. [10]Increased incidence of acute rejection episodes correlates well with high PRA reactivity. [10] Panel reactive antibodies have been the main method for detecting sensitization since 1960s. Recent changes in the technique of PRA has been introduced as calculated PRA, which helps to facilitate transplant of highly sensitized patients. [3]However, unfavorable graft outcomes including acute and chronic rejection still happen, even with HLA-matched donors and recipients. As a result, researchers have paid attention to other complementary factors, such as donor-specific antibodies, to predict transplant rejection. Current debates regarding the potential value of such complementary factors in predicting graft outcome have convinced transplant specialists to search for other noninvasive immunologic markers such as serum soluble CD30 (sCD30), which can be used as a predictor of graft rejection. [4]In our study The etiology of ESRD of the participating patients in our study revealed that the commonest cause of ESRD was unknown in 17 cases (42.5%), followed by FSGS in 7 cases (17.5%), then HTN and vesicoureteric reflux were in 4 patients (10%). Shooshtarizadeh et al., reported that the causes for end-stage renal disease were mainly diabetes mellitus, hypertension, and obstructive uropathy. [11] also the same finding was reported by Azarpira et al., the original cause of End stage Renal Disease was 27 of them was Diabetic Nephropathy, 10 of them were Hypertension,6 were Polycystic kidney disease and 5 of them were unknown cause. [12]In our study the mean±SD value of time of hot ischemia per minutes for all patients was 3.35±0.84, and the mean±SD value of time of total ischemia per minutes for all patients was 45.68 ±16.61. However, Azarpira et al., study 2010 reported in his study that the mean value of Cold ischemia time was 10.2 ± 3.1. [12]In our study the mean±SD values of serum creatinine before transplantation was 9.45±1.98 mg/dl and serum creatinine after transplantation mean ±SD was 1.21±0.42 mg/dl. The mean value of CD30 level day 0 before transplantation which was 113.5 (86.5-162.5), and the median value of CD30 values day 30 after transplantation was 43 (31.71-69) which is in agreement of Süsal et al., study that was performed on 409 patients, median of serum creatinine on post-transplant day 30 was 1.8 mg/dL, there was no association between serum sCD30 and S.creatinine was observed. [13] While in Valke et al. study the median of sCD30 concentration before transplantation was 126.8. [14]In our study there was no significant relation between PRA positivity and high sCD30 level (P =.199). Pre-transplant sCD30 concentrations were not significantly different between men and women (median value 110.5 vs 170 with P value 0.112). Pre- and posttransplant sCD30 levels significantly correlated with each other (P = 0.000). Serum soluble CD30 levels after transplantation were significantly higher in younger patients (r =-0.324, P value = 0.041), soluble CD30 levels were significantly higher in patients with increased serum creatinine after transplantation (P-value 0.001), and finally Cd30 after transplantation were higher in patients with long transient ischemia during operation (P-value 0.024). which is In agreement with Shooshtarizadeh et al., study which reported that there was no significant relation between PRA positivity and high sCD30 level (P =.714). Pre-transplant sCD30 concentrations were not significantly different between men and women (95.8 ±51.8 vs 83.3 ± 29.9; P =. 261). Pre- and posttransplant sCD30 levels significantly correlated with each other (r = 0.535, P = 0.001). Serum soluble CD30 levels were significantly higher in patients younger than 40 years versus those older than 40 years (107 ± 59.1 vs 79.9 ± 28.1; P =.008). [11] And in agreement with Azarpira et al, study which reported that sCD30 level was 130.3 ± 81.1 U/ mL (range 39- 400 U/mL). There was no significant difference in sCD30 level with respect to the gender of the recipient. [12] Also in agreement with Trailin et al., pre-transplant serum sCD30 levels ranged from 9.1 to 169.6 U/mL with a median value of 49.5 U/mL, and they were higher in younger patients (r = -0.373, P = 0.012) as well as in the recipients of living-donor graft (rho = -0.312, P = 0.037). There was a significant decrease of post-transplant sCD30 values (P < 0.01) which had no relation with the donor age. However, there was a median decline more in the recipients of grafts from younger donors (-54.46%) than from older one (-27, 31%), P = 0.019.it was observed that recepients with deceased donor graft showed a significant decrease in SCD30 levels post transplant (P < 0.001). SCD30 declined significantly post-transplant both in male and in female (P < 0.001), and the median decline was more evident in male: -52.68% than in female: -24.02% (P = 0.053). [15] Lopez-Hoyos et al., agreed with our study in that Serum levels of sCD30 decreased significantly in samples Post-transplantation (1 months: 12.5 _ 10.1 ng/mL) compared with pre-transplantation (19.4 _ 6.9 ng/mL; P _.001. [16] Also in Trailin et al., posttransplant sCD30 values decreased in most patients and its level was from 8.8 to 56.7 U/mL with a median value of 23.1 U/mL, and they were not affected by baseline recipients and donors characteristics. [15] In agreement with Azarpira et al., the high sCD30 group showed a higher incidence of acute rejection within the post-transplant one month than the low sCD30 group. However, this was not statistically significant (41% versus 33%, P> 0.05). One patient with received antithymocyte globulin (OKT3) and had high pre transplant sCD30 (128 IU/mL) levels. [12] In Süsal et la., Forty-two percent of patients with high sCD30 on post-transplant day 30 (≥40 U/mL) demonstrated a high pre-transplant sCD30 value of more than or equal to 100 U/mL (_the relevant pre-transplant cut-off established in previous studies), in contrast to only 11% of patients with low sCD30 on day 30 (P_0.001), thus indicating a strong association between pre- and post-transplant sCD30 levels. [13] Cervelli et al., found a strong relation between Post-transplant levels of sCD30 and acute rejection risk. They noticed that if sCD30 concentration declined after immunosuppressive therapy, then the rate of acute rejection would reduce dramatically, while failure of sCD30 levels to decrease after immunosuppression would lead to a high probability of acute rejection, despite proper administration of immunosuppressive drugs. They suggested that patients with increased pre-transplant and post-transplant levels of this marker should receive stronger immunosuppressive regimens to properly protect their grafts against immunologic host response. [17] On the other hand, some studies did not find any correlation between sCD30 level and occurrence of acute rejection. Azarpira et al., were unable to find any relation between pre-transplant or 5-day post-transplant sCD30 levels, graft survival, or incidence of acute rejection. [12] Interestingly, in disagreement with Nafar et al., that did find a significant relation between Post-transplant sCD30 day 30 levels and acute rejection; however, they failed to show such a relation with pretransplant sCD30 levels. [18] Furthermore, in their study, there was no statistically significant difference between pretransplant and posttransplant sCD30 levels (58 ± 52 ng/mL vs 55 ± 49 ng/mL). [18] In disagreement with Yang et al., that showed a similar relation between pre-transplant and Post-transplant sCD30 and the incidence of acute graft rejection episodes. Their study suggested that the higher the pre-transplant and 7-day post-transplant sCD30 levels were, the more was the probability of graft rejection. However, that study could not find any significant relation between sCD30 levels measured 1 month after transplant and graft rejection. [19]In our study the cutoff point of CD30 was determined by >75ngm/ml, and the area under curve was 0.886 means cut off point is trusted by 88,6%, and the CD30 marker is sensitive by 80%, and specific by 85.7% with positive predictive value 44.4% and negative predictive value 96.8%.In comparison of our study, a cutoff of 100 U/mL is used to differentiate between high- and low-risk patients in many similar studies Therefore, at a cutoff of 100 U/mL, the sensitivity, specificity, positive predictive value, and negative predictive value of pretransplant serum sCD30 levels to predict acute rejection were 70%, 74.6%, 29.1%, and 94.3% [11].There are some studies that find different cutoffs as the optimal discriminating value. For instance, Nafar et al. [18] and Kamali et al. [20], in 2 separate studies, determined the optimal cutoff to be 20 U/mL and 41 U/mL. In Giannoli et al., study 2017 examining the distribution of sCD30 values among patients with and without acute rejection, they noticed that pre-transplant sCD30 level was a sensitive tool to predict acute rejection at low cutoff points, and at best scenario (ie, a cutoff of 60 ng/mL), the test had a sensitivity of 80%. On the other hand, it was a specific test, with a specificity greater than 90% at higher cutoff (ie, 140-150 ng/mL), and in this circumstance, it had a low sensitivity. [21] Lower pre-transplant sCD30 showed reasonable discriminatory power in predicting the slow graft function: AUC = 0.781, CI: 0.613 – 0.948, P = 0.023, and derived the cut-off value of 60.1 U/mL from the ROC-curve (see Figure 1), below which sCD30 exhibited the highest sensitivity for SGF (100%). [15]
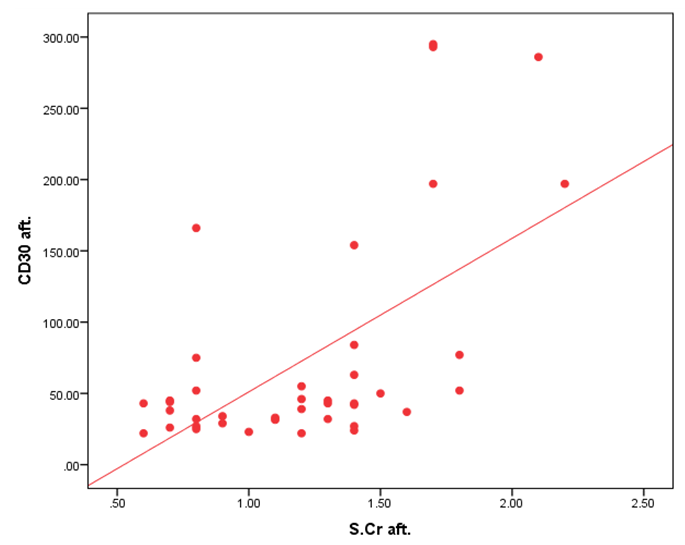 | Figure 1. Correlation between CD30 day 30 and S.cr after Transplantation |
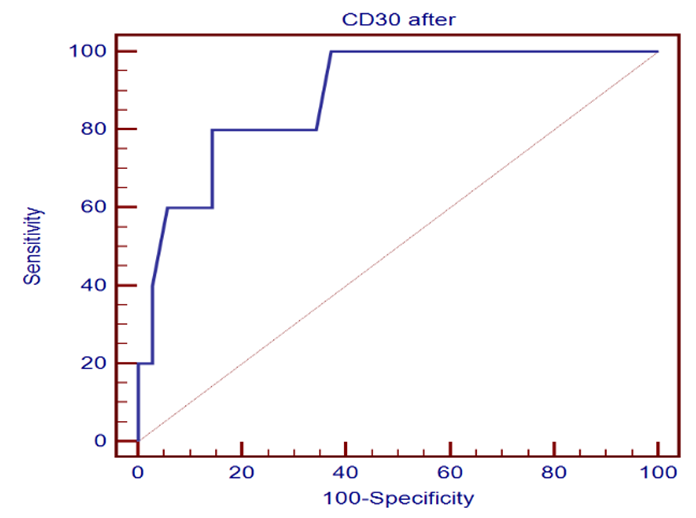 | Figure 2. Sensitivity and specificity of CD30 marker |
5. Conclusions
- Our study shows a direct relation between pre-transplant sCD30 levels and post-transplant serum creatinine levels day 30. We did not find any significant relation between PRA positivity and sCD30 values. This may be because of the relatively low level of PRA in our transplanted patients, that is, actually no recipient had a PRA higher than 20%. Our study had few limitations. This study referred to a limited number of patients, sCD30 levels as a predictor marker have only been determined once per patient and pre-transplantation accompanying post transplantation monitoring of sCD30 levels over time has not been performed. Therefore, whether CD30 molecule is involved in acute allograft rejection and its role in allogeneic immune response needs furtherf investigation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML