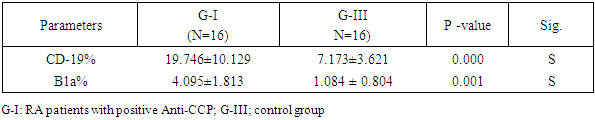-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2019; 9(3): 47-54
doi:10.5923/j.cmd.20190903.02

The Pathogenic Role of B1a Lymphocyte in Rheumatoid Arthritis Patients and Its Relation with Seropositivity
Zeinab H. El Sayed1, Hanan A. EL-Hagrasy2, Dina Badway2, Maha Salah Eldin Mohamed3
1Department of Internal Medicine, Faculty of Medicine, Al-Azhar University for Girls, Cairo, Egypt
2Department of Clinical Pathology, Faculty of Medicine, Al-Azhar University for Girls, Cairo, Egypt
3Department of Rheumatology and Rehabilitation, Faculty of Medicine, Al-Azhar University for Girls, Cairo, Egypt
Correspondence to: Zeinab H. El Sayed, Department of Internal Medicine, Faculty of Medicine, Al-Azhar University for Girls, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

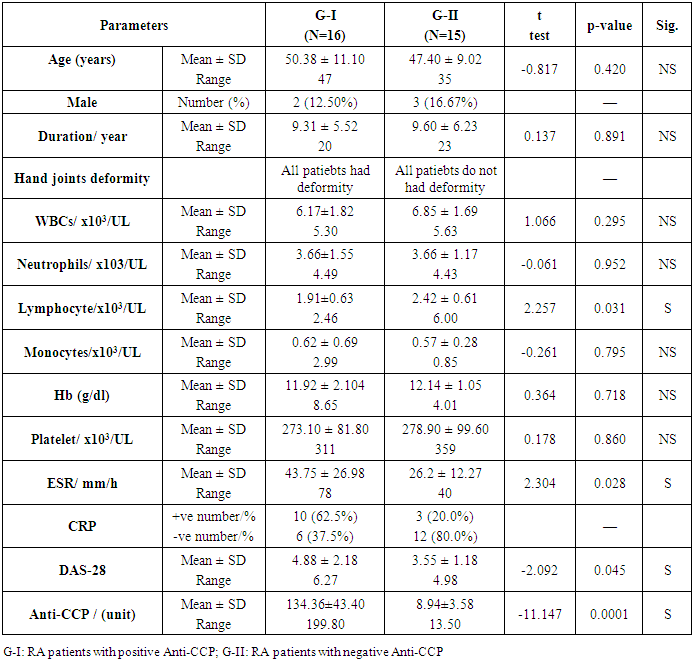
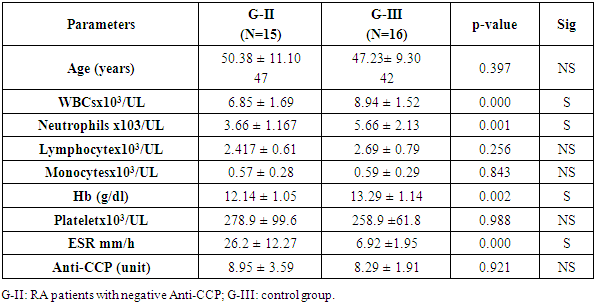
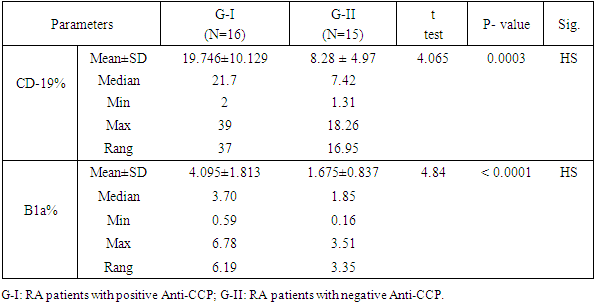
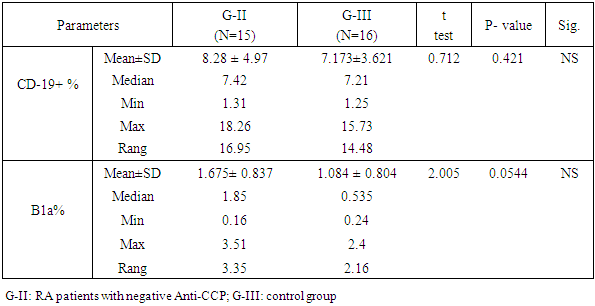
Objectives: Patients with Rheumatoid arthritis (RA) have immunological abnormalities encompass T and B lymphocytes cell dysfunctions which lead to the production of pathogenic autoantibodies. According to the presence or absence of CD5 B1 lymphocyte cells are subdivided into: B-1a (CD5+), which participate in the innate immunity and B-1b (CD5-), which involved in acquired immunity. The B-2 lymphocytes are known to play an important role in the development of RA. The aim of the study is to assess the frequency of circulating CD5+ CD19+ B1a lymphocytes in Anti-cyclic-citrullinated protein antibody (Anti-CCP) positive versus Anti-cyclic- citrullinated protein antibody (Anti-CCP) negative in RA patients. Methods: In a prospective study which included 16 RA patients with positive Anti-citrullinated protein antibody and 15 patients with negative Anti-citrullinated protein antibody. Another group consisted of 16 apparently healthy subjects serving as a control group. The CD5+ CD19+ B1a lymphocyte cell in blood levels in patients with RA and normal controls was measured using flow cytometry. Results: In a comparison between RA patients with +ve ACPA and RA patients with Anti-CCP –ve groups, we showed a significant elevation in Anti-CCP (P=0.0001), CD19+% (p-value =0.0003), and B1a% lymphocyte (p-value< 0.0001) in RA patients with positive Anti-CCP. Also, there was a significant elevation of ESR (p-value= 0.028), and in DAS-28 (P = 0.045) in RA patients with positive Anti-CCP. But we found a significant reduction of absolute lymphocyte counts (p=0.031) and RA patients with positive Anti-CCP than RA patients with negative Anti-CCP. There was no significant difference of Anti-CCP (p-value= 0.921), CD-19+ % (p-value= 0.421), B1a % (p-value= 0.0544), or absolute lymphocyte cell count (p-value= 0.256) when compared between RA patients with -ve Anti-CCP group and control group. However, we found a significant reduction of WBCs (p-value= 0.000) in RA patients with –ve Anti-CCP group and control group. There were significant elevation of both CD19+ % (p-value= 0.000), and B1a (p-value= 0.001) in RA patients with +ve Anti-CCP group in comparison to the control group. Conclusion: Our result reported that the frequency CD5+ CD 19+ B1a lymphocyte in peripheral blood were elevated in rheumatoid arthritis patients with positive Anti-CCP. Therefore this study adds to the growing evidence of CD5+ CD 19+ B1a lymphocyte in the pathogenesis of seropositive rheumatoid. Recommendation: Therefore, targeted elimination of the CD5+ CD19+ B1a lymphocyte in RA patients mainly who had Anti-CCP postive may be useful for treatment.
Keywords: B1a lymphocyte, CD5+ CD19+ B1a cell, Cluster of differentiation, Rheumatoid arthritis, Sero-positivity
Cite this paper: Zeinab H. El Sayed, Hanan A. EL-Hagrasy, Dina Badway, Maha Salah Eldin Mohamed, The Pathogenic Role of B1a Lymphocyte in Rheumatoid Arthritis Patients and Its Relation with Seropositivity, Clinical Medicine and Diagnostics, Vol. 9 No. 3, 2019, pp. 47-54. doi: 10.5923/j.cmd.20190903.02.
Article Outline
1. Introduction
- Rheumatoid arthritis (RA) is a globally-prevalent autoimmune disease that affects around 1-2 % of the population with a predilection to females. Although the pathological hallmarks of RA are established as chronic synovial joints inflammation and symmetric progressive joint damage, the involved mechanisms in these processes remain elusive. Anti-Cyclic citrullinated antibody (Anti-CCP) is an autoantibody that is present in the majority of the patients with RA [1]. The Anti-CCP yield good prognostic performance in predicting disease severity and response to treatment [1]. The B-cell compartment was classified at least into two main subsets: B-1 cells; and B-2 cells, which differ from each other in the developmental origin, surface marker expression, and functions [2]. B1 cells are the spontaneous secreting type that delivers antibodies, even in the absence of infection or immunization, such as rheumatoid factor and Anti-CCP. Moreover, they have been shown to secrete interleukin 10 (IL-10) in mice [2] [3] [4]. The B-1 lymphocytes under further subdivision according to the presence or absence of trans-membrane molecule CD5 into (1) B-1a (CD5+), which participate in the innate immune response to infections and play a central role in immunoregulation, (2) B-1b (CD5-), which involved in acquired immunity [4]. CD-19 is a surface biomarker for mainly mature B lymphocyte with higher expression in B1 than B2 lymphocyte and is considered a pan B cell marker. In autoimmune diseases, the relationship between the increased level of autoantibodies and CD5+ B lymphocyte is established [5]. The aim of the study is to assess the frequency of circulating CD19+ CD5+ B1a lymphocytes in Anti-citrullinated protein antibody positive verses Anti-Cyclic citrullinated antibody negative in RA patients.
2. Patients and Methods
- Patients: The current study included 31 Egyptian patients with RA studied. There were collected from the outpatient clinics of physical and internal medicine departments, Al-Zahraa University Hospital, Cairo, Egypt between October 2018 and January 2019. Based on Anti-CCP positive or negative, the patients were subdivided into two groups: G-I included 16 RA patients with positive Anti-CCP and G-II: included 15 RA patients Anti-CCP were negative. Other 16 apparently healthy subjects (age and sex-matched) were enrolled in our study as a control group (G–III). This study was done according to the ethical committee of the Faculty of Medicine, Al Azhar University. The study was conducted in accordance with the Declaration of Helsinki [6], and informed consent was taken from every participating individual.Exclusion criteria:1. Rheumatoid arthritis patients had other autoimmune diseases or ischemic heart disease.2. Any patients had spondyloarthritis. The study groups:• Group-I: Included 16 RA patients with Anti-CCP positive• Group-II: Included 15 RA patients with Anti-CCP negative• Group-II: Included 16 healthy subjects All studied participants were subjected to the following:Clinical history and thorough examination were done. Disease activity scoring 28 (DAS 28) [7] was done for each patient and ESR (Westergren method) [8]. was also done. Laboratory investigations included: Routine laboratory investigations including full blood count, ESR, C-reactive protein test (positive or negative), Rheumatoid factor, double stranded-DNA antibodies were done for each patient and control subjects. Also, Anti-CCP [9], CD19 %, CD5 %, and B1A % [10] were done for each patients and control subjects. The radiological study included plain chest x-ray, all joints Palin X rays, abdominal ultrasound, echocardiography, and ECG.Methodology: Complete blood count (CBC): 10 ml of venous blood from all subjects was extracted: 5 ml into an EDTA tube to measure CBC, ESR, and B1a, while 5 ml was centrifuged and the collected serum was divided into two aliquots for CRP and Anti-CCP measurements.Using automated CBC machine Cell Dyn Ruby, C. Reactive protein (CRP) by latex, Anti-CCP -IgG estimation by ELISA technique. We measured Anti-CCP -IgG level by ELISA technique using QUANT Lite (Inova Diagnostics Inc. San Diego, CA USA 92131kit lot#038035), according to the manufacturer's instructions), and B1a% measurement using flow cytometry. Flow Cytometry assay: We performed flow cytometry measurements, using 4-color FACS Calibur (BD, Biosciences, San Jose, USA) in the Clinical Pathology Department, Al-Zahraa Hospital, AL-Azhar University. Then, the CellQuest-Pro software (BD Biosciences, San Jose, USA) was used for data analysis. Fifty micron of fresh sample was incubated with 5 micron of the following fluorochrome-conjugated antibodies: PE-conjugated anti-human CD19 (BD Biosciences, USA, cat555413, lot; 5274713) and FITC-conjugated anti-human CD5 (BD Biosciences, USA, cat; 555352 lot 5093682). Initial gating was performed by typical forward and sideways scatter on mature lymphocytes area (figure, 1A). Isotype-matched control antibody was used to set-up the cutoff of positivity (figure 1 B), then B1a cells were detected on double parameter plot in the upper right quadrant, representing an area of double positivity of both markers (CD19 represented on X-axis and CD5 marker represented on the Y-axis) for both cases and controls(figure 1C and 1D).
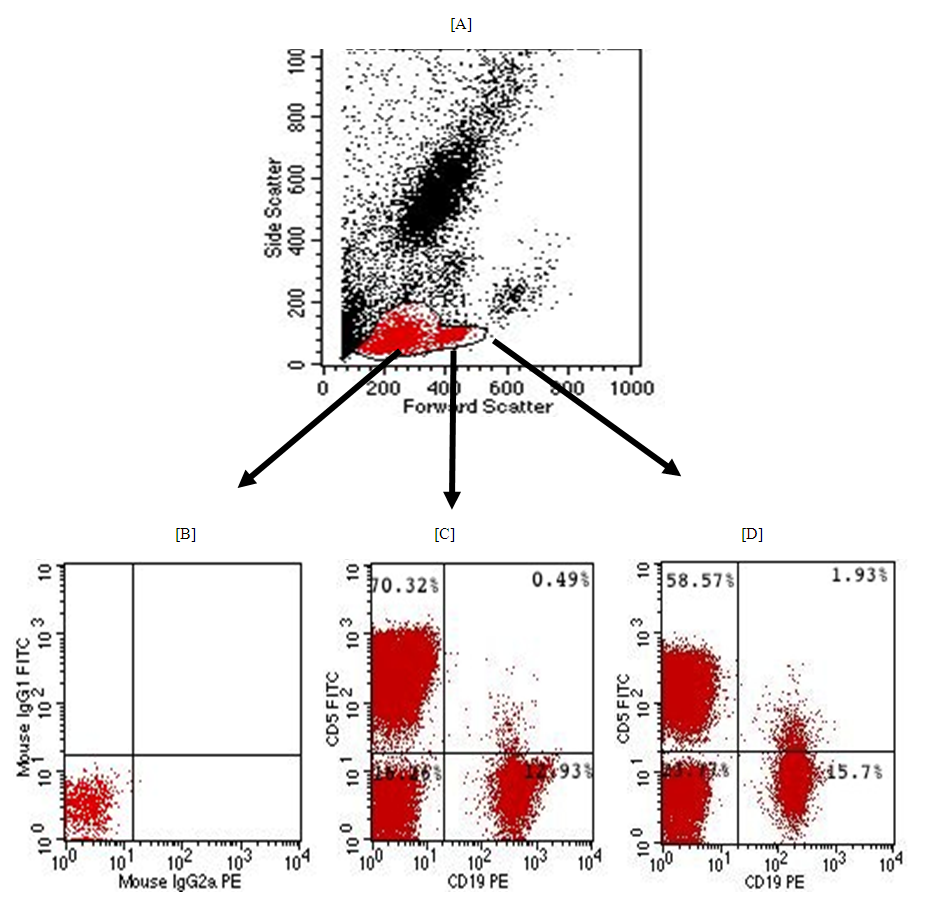 | Figure 1. Illustrating gating strategy for detection of B1a lymphocyte cells |
3. Results
- Thirty-one RA patients and 16 controls were enrolled in the current study. The distribution of the patients based on the serum level of Anti-CCP, as shown in table 1 and fig.2.
|
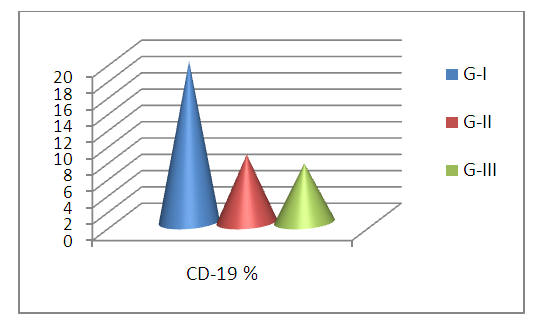 | Figure 2. CD-19 % in G-I (RA with positive Anti-CCP), G-II (RA with negative Anti-CCP), and G-III (control group) |
|
|
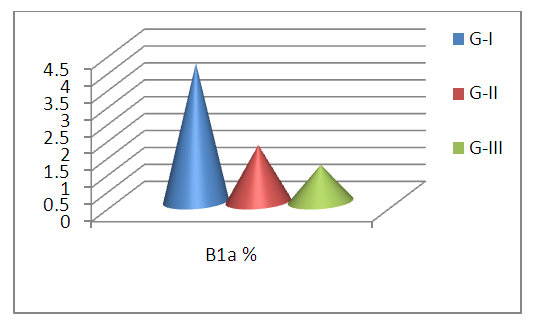 | Figure 3. B1a % in G-I (RA with positive Anti-CCP), G-II (RA with negative Anti-CCP), and G-III (control group) |
|
|
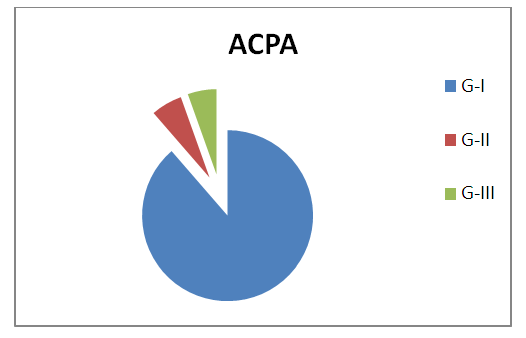 | Figure 4. Serum Anti-CCP level concentration in G-I (RA with positive Anti-CCP), G-II (RA with negative Anti-CCP), and G-III (control group) |
4. Discussion
- Dysregulation of B1 lymphocyte function has been involved in many autoimmune diseases. In the past two decades, the role of B lymphocytes in RA pathogenesis became clear. Therefore, researchers considered targeting them using monoclonal antibodies (mAb) [11]. The first in this regard were CD20 monoclonal antibodies that targeted mainly mature B lymphocytes, such as rituximab [12] and ocrelizumab [13]. However, CD20 mAb does not deplete pre-B or immature B cells and certain peripheral B cell subpopulations. Therefore, researchers considered targeting CD19 marker (a broad biomarker for B lymphocytes) using CD19 mAb, which showed success in animal studies [14]. The aim of the study is to assess the frequency of circulating CD19+ CD5+ B1a lymphocytes in Anti-citrullinated protein antibody positive verses Anti-Cyclic citrullinated protein negative in RA patients. In our study, we found that the patients with rheumatoid arthritis and anti-cyclic citrullinated protein positive have a significantly elevated level of circulating B1a lymphocyte expressing CD 5+ and CD 19+ marker compared to normal control (table 5). These findings suggested that there is a proliferative expansion of circulating CD 5+ B1a lymphocytes in RA patients and it has a role in the pathogenesis of the development of rheumatoid arthritis. This result is in agreement with Lu et al, 2016 [15] who reported that the peritoneal CD5+ B1a lymphocyte undergoes proliferation and transfer into the peripheral lymphoid organs and inflamed joints. Another study suggested that there is a functional change of B1a lymphocyte may result from their migration to the peripheral lymphoid organs and to the sites of inflammation during autoimmunity [16]. Similarly, a study by Jiménez-Alonso and his colleagues 2002 [17] found that the mean of CD5+ B lymphocyte level in the peripheral blood was significantly higher in uveitis patients than the control group and also they found significant elevation of CD5+ B lymphocyte level in different subgroups of uveitis than the control group. Jensen and his colleagues 2012 [18] reported that CD19+ CD5+ B1a cell previously was committed to natural antibody production, also it can be driven by antigens to produce various high-affinity autoantibodies, some of these autoantibodies are dangerous for pregnancy because these can reach placenta causing pre-eclampsia. So B1a lymphocyte cells not only contribute to the pathogenesis of autoimmune disease but also migrate to the site of inflammation to exacerbate the symptoms. Furthermore, the mean (SD) of CD19+ CD5+ B1a cells in the circulating blood was found a significant increased in rheumatoid arthritis patients with anti-cyclic citrullinated protein antibodies positive group than the rheumatoid arthritis patients with anti-citrullinated protein antibodies negative group (table 3). However, we found no significant difference in CD5+ CD19+ B1a cells between rheumatoid arthritis patients with anti- cyclic citrullinated protein negative and control group.Sokolove and his colleagues 2014 [19] study reported that a significant elevation of disease activity as an elevation of systemic inflammatory markers was associated with the presence of anti-citrullinated protein antibodies which indicate that anti- cyclic citrullinated protein may have directed contribute to the pathogenesis of rheumatoid arthritis. On the vise versa, in the rheumatoid arthritis patients with anti- cyclic citrullinated protein, the negative group had the CD5+ C19+B1a with no difference in comparison with the control group. It is explained by the anti-cylic citrullinated protein were secreted by CD5+ C19+B1a cells [20].Our study showed that there was a significant decrease of mean (SD) of absolute lymphocyte cells count in rheumatoid arthritis patients with anti- cyclic citrullinated protein positive group than the rheumatoid arthritis patients with anti- cyclic citrullinated protein negative group (table 1) despite the mean (SD) of white blood count between the same groups which was no significant (table 1). Furthermore, we found no difference of absolute lymphocyte cells count between rheumatoid arthritis patients with anti- cyclic citrullinated protein negative group and control group (Table 2). This means that in the rheumatoid arthritis with Anti-CCP positive have less absolute lymphocyte cells count also have more immune disturbance in the same time have elevated CD5+ CD19+ B1a lymphocyte can be explained by the other subset types of lymphocyte cells decrease in seropositive rheumatoid patients as CD8+ cytotoxic T lymphocyte, CD3+, CD65+ and CD16+ T lymphocyte [21]. Some studies reported that the functional changes occur in B1a lymphocyte cells resulting from their aberrant run away to the peripheral lymphoid organs and site of inflamed joints on autoimmunity. Also, found CD5+ CD19+ secreted low level of granulocyte-monocyte-colony stimulating factor [22]. Another study reported that the CD5+ Cd19+ B1a lymphocyte was found in healthy subjected in very small percentage as well as in chronic lymphocytic leukemia and it has the ability to self-renewal for life with increased the risk of dysregulation [23]. Furthermore, CD19 is the key signaling component for the multi-molecular cell surface signal-transduction complex. Moreover, it links the innate and adaptive immune responses due to its subsequent signaling with CD21, a receptor for C3 complement fragments. The CD19 cytoplasmic component entails 9 tyrosine residues, that -upon phosphorylation after CD19 receptor binding- recruit regulatory molecules to the cell surface. These regulatory molecules include lyn and other src-family protein tyrosine kinases, which strengthens the signals generated by the B-cell antigen receptor. Reduction in the cell surface density in transgenic mice led to hypo-responsive B cells with deficient humoral responses. On the other hand, CD19 over-expression increased the responsiveness of B cells to transmembrane signals [24]. Therefore, it seems that CD19 regulates the expansion and function of peripheral B lymphocytes. Further, B1a cells have been found to promote Th1 and Th17 cell differentiation and inhibit Treg cell differentiation [25,26]. Mantovani and colleagues found that human CD5+ B cells (B1a) make somatic hyper-mutated IgM rheumatoid factors [20].
5. Conclusions
- Our result reported that the frequency CD5+ CD 19+ B1a lymphocyte in peripheral blood were elevated in rheumatoid arthritis patients with positive Anti-CCP. Therefore this study adds to the growing evidence of CD5+ CD 19+ B1a lymphocyte in the pathogenesis of seropositive rheumatoid.
6. Recommendations
- Therefore, targeted elimination of the CD5+ CD19+ B1a lymphocyte in RA patients mainly who had Anti-CCP postive may be useful for treatment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML