-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2019; 9(2): 26-35
doi:10.5923/j.cmd.20190902.02

Semen Profile of Men Presenting with Infertility at First Fertility Hospital Makurdi, North Central Nigeria
James Akpenpuun Ikyernum1, Ayu Agbecha2, Stephen Terungwa Hwande3
1Department of Medical and Andrology Laboratory, First Fertility Hospital, Makurdi, Nigeria
2Department of Chemical Pathology, Federal Medical Centre, Makurdi, Nigeria
3Department of Obstetrics and Gynaecology, First Fertility Hospital, Makurdi, Nigeria
Correspondence to: Ayu Agbecha, Department of Chemical Pathology, Federal Medical Centre, Makurdi, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: There is a paucity of information regarding male infertility, where data exist, fertility estimates heavily depends on socio-demographic household surveys on female factor. In an attempt to generate reliable male infertility data using scientific methods, our study assessed the seminal profile of men. Aim: The study aimed at determining the pattern of semen profile and its relationship with semen concentration in male partners of infertile couples. Materials and Methods: the cross-sectional study involved 600 male partners of infertile couples from June 2015 To December 2017. Frequency percentages of socio-demographics, sexual history/co-morbidities, and semen parameters were determined. The association of semen concentration with other semen parameters, socio-demographics, and sexual history/co-morbidities was also determined. Results: asthenozoospermia and teratozoospermia were the commonest seminal abnormalities observed, followed by oligozoospermia and azoospermia. Primary infertility (67%) was higher than secondary infertility (33%). Leukospermia was observed in 58.5% of male partners. No significant (p>0.05) association was observed between socio-demographics, co-morbidities, and semen concentration. History of sexually transmitted infections (p<0.05) and other semen parameters (p<0.005) were significantly associated with semen concentration. Conclusion: Our study found significant abnormal semen quality, which may contribute to male factor infertility. Scientific studies are needed to investigate factors causing the high rate of primary male infertility and male infertility in our environment. This will enable infertile couple’s access treatment to improve fertility rates.
Keywords: Male infertility, Asthenozoospermia, Teratozoospermia, Oligozoospermia, Azoospermia
Cite this paper: James Akpenpuun Ikyernum, Ayu Agbecha, Stephen Terungwa Hwande, Semen Profile of Men Presenting with Infertility at First Fertility Hospital Makurdi, North Central Nigeria, Clinical Medicine and Diagnostics, Vol. 9 No. 2, 2019, pp. 26-35. doi: 10.5923/j.cmd.20190902.02.
Article Outline
1. Introduction
- Infertility is a worldwide problem defined by the world health organization (WHO); clinically as “a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse” [1] and epidemiologically as “women of reproductive age (15–49 years) at risk of becoming pregnant (not pregnant, sexually active, not using contraception and not lactating) who report trying unsuccessfully for a pregnancy for two years or more” [2]. The WHO further classifies infertility into primary “When a woman is unable to ever bear a child, either due to the inability to become pregnant or the inability to carry a pregnancy to a live birth” and secondary “When a woman is unable to bear a child, either due to the inability to become pregnant or the inability to carry a pregnancy to a live birth following either a previous pregnancy or a previous ability to carry a pregnancy to a live birth” [3]. A comprehensive data based on systematic analysis of 277 health surveys from 1999 to 2012 reports that at least 50 million couples worldwide experience infertility [4]. A further breakdown in the trend of infertility globally revealed that Infertility prevalence was highest in South Asia, Sub-Saharan Africa, North Africa/Middle East, Central/Eastern Europe and Central Asia [4]. Most of the demographic and reproductive household survey data used in determining and estimating global trends of infertility are based on the female factor as defined by WHO [4-6]. Currently, information on global male infertility is lacking and this paucity of data is worse for developing countries [7] like Nigeria. In some of these regions, male fertility rates are estimated from female infertility statistics. Data indicates the global prevalence of male infertility as 2.5% to 12% with 2.5%-4.8% infertile men in sub-Saharan Africa. This figure is relatively low compared to Europe (7.5-12%) where infertility rates are reportedly believed to be more accurate. The calculated primary male infertility rate stood at 0.4-0.8%, while secondary male infertility was 2.32-4.4%. The low male infertility rate observed in the region is ascribed to underreporting [7]. A worldwide decline in sperm count has been presented vastly in the past few decades by several studies [8, 9]. Over the past decades, there has been a progressive decline in sperm concentration in Nigerian males [10-12].Relatively little is known about the specific risk factors for the prevalence of male infertility around the world. However, several reports exist on the causes and risk factors of male infertility in the African population. These include the effects of occupational and environmental exposure to physical or chemical agents, infections, Dietary and sedentary lifestyles, excessive alcohol consumption, cigarette smoking and genetic factors [12-14].The desire to have children by couples, together with the evolution of assisted reproductive technology, more male partners of infertile couples avail themselves for fertility checks. Seminal fluid analysis however not the gold standard method has shown to be a reliable surrogate method for investigating male fertility status in both clinical and research settings [15].Semen profile in the context of this present study refers to the biological, qualitative and quantitative features of seminal fluid. A standardized detailed semen analysis procedure with references is provided in the WHO laboratory manual for the examination and processing of human semen [15]. WHO 2010 reference values for seminal parameters include: Normal sperm parameters; Semen volume greater than or equal to 1.5 ml, pH value of greater than or equal to 7.2, total spermatozoa greater than 15 x106 cell/ml, progressively motile spermatozoa greater than 32%, morphologically normal spermatozoa greater than 4%, leukocytes less than 1x106 cells/ml. For the purpose of this study, the following terms were used, and their meanings were stated in the table below:
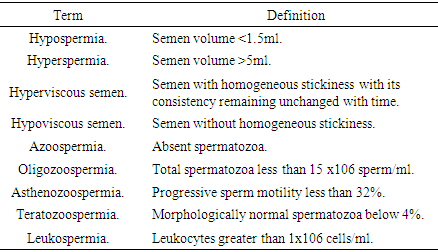 Few studies have assessed sperm quality in Nigerian men in an attempt to avail data on male infertility. Most of these studies were not carried out at fertility hospitals and as such lacked equipment and expertise in assessing fertility issues. Our study was carried out in a hospital specially designed to meet the needs of the study population, therefore adhered strictly to standard conditions. The study aimed at determining the pattern of semen profile and its relationship with semen concentration in male partners of infertile couples.
Few studies have assessed sperm quality in Nigerian men in an attempt to avail data on male infertility. Most of these studies were not carried out at fertility hospitals and as such lacked equipment and expertise in assessing fertility issues. Our study was carried out in a hospital specially designed to meet the needs of the study population, therefore adhered strictly to standard conditions. The study aimed at determining the pattern of semen profile and its relationship with semen concentration in male partners of infertile couples.2. Materials and Methods
2.1. Study Design and Population Sampling
- The cross-sectional study assessed the quality of semen in male partners of infertile women. After obtaining institutional ethical clearance, a total of 600 consenting male partners of the infertile couple were recruited from June 2015 To December 2017. The bio-data and relevant history of the patients including their reproductive lives were obtained. After abstinence from ejaculation for 3-5 days, semen sample was collected from the participants into a sterile screw-capped universal plastic container in a side room next to the laboratory. Spilled sample collection was avoided and participants who did not consent and adhere to the abstinence criteria were excluded from the study.
2.2. Study Area
- First Fertility hospital is among the few specialist fertility clinics in Nigeria. The hospital is located in Makurdi the capital of Benue State North Central region of Nigeria. Due to the unavailability of such specialist institutions, the clinic renders fertility services to the entire region of Nigeria. Most of the assisted reproductive technology births in the region are done in this facility. The facility is equipped with state of the art equipment, manned by specially trained obstetricians/ gynecologists, embryologists, andrologists and medical laboratory scientists in the area of fertility science.
2.3. Laboratory Methods
- Semen analysis was carried out in the andrology laboratory of the hospital following the WHO 2010 procedure. The seminal fluid analysis was done paying attention to semen volume, viscosity, liquefaction, pH, appearance, sperm concentration, motility, and morphology [15]. Microscopic examination of seminal fluid was conducted by a certified scientist/andrologist using a trinocular microscope and a Makler counting chamber under a laminar hood.
2.3.1. Semen Analysis
- All semen samples were received in the laboratory within 30 min of production. After liquefaction for 30 min or a maximum of 60 min, semen samples were evaluated for sperm concentration, motility, and morphology. The volume of the ejaculate was determined by aspirating the liquefied sample into a graduated disposable pipette. Sperm counting and motility assessment were performed using the counting chamber. A volume of 3–5 μL of semen sample was transferred to the center of the chamber. Sperm count was performed in 10 squares of the chamber. The total sperm count is the end concentration expressed as 106 spermatozoa/ml. Sperm motility was assessed in 100 random spermatozoa by characterizing them as rapidly forward fast progressive motility and immotile/no movement, and the motility was expressed as a percentage. A total of 200 sperm cells were characterized as morphologically normal or abnormal and the final morphology was expressed as a percentage.
2.4. Statistical Analysis
- All statistical analyses were performed using the IBM Armonk, New York, United States SPSS version 21. Data were presented as percentage frequency distribution. Associations between variables were determined using Chi-squared and Fisher's exact test, with the level of significance set at less than 0.05 (P < 0.05).
3. Results
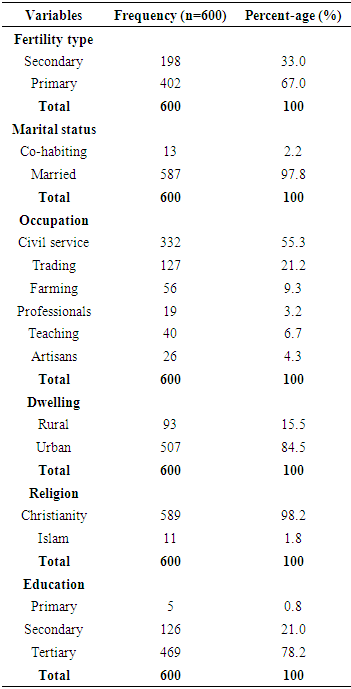
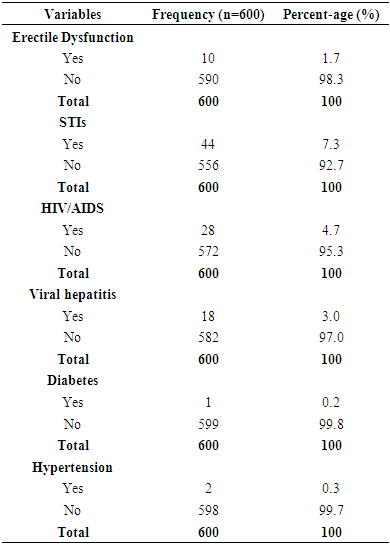
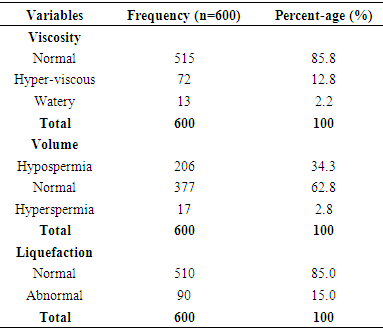
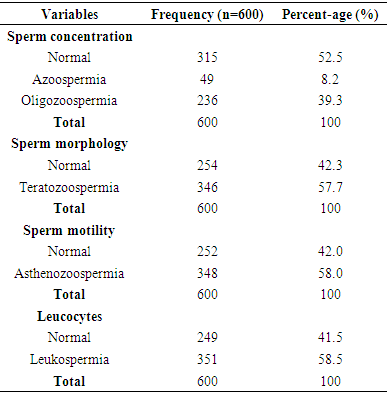
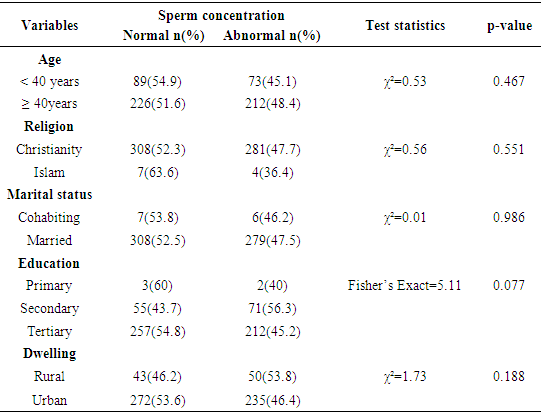
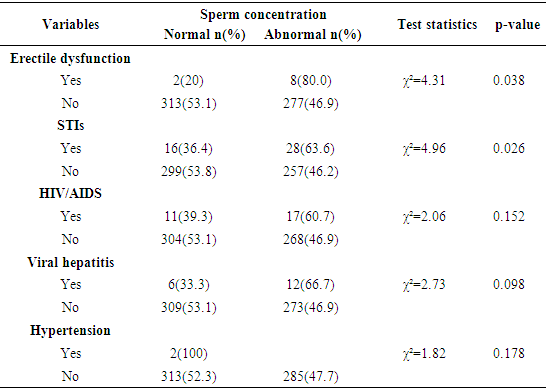
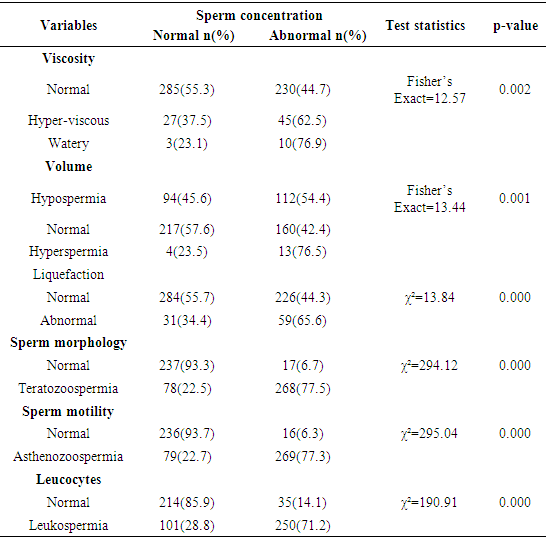
- The frequency percentages of socio-demographic characteristics, sexual history and co-morbidities of the study participants are summarized in table 1 and 2 respectively. Of the overall 600 males studied, 402 (67%) couples had primary infertility while 198(33%) had secondary infertility. Majority of the participants; were married (97.8%) compared to co-habiting (2.2%) partners, had normal erections (98.3%) compared to men with erectile dysfunction (1.7%), had normal ejaculation (100%). Majority of the study participants had no sexually transmitted infections-STIs (92.7%), HIV/AIDS (95.3%), viral hepatitis (97%), diabetes (99.8%) and hypertension (99.7%) compared respectively (7.3%, 4.7%, 3.0%, 0.2%, 0.3%) to their diseased counterparts. The frequency percentage of the macroscopic semen profile is presented in table 3. A greater percentage of the study participants had normal semen viscosity (85.8%) and liquefaction (85.0%). Whereas 62.8% had normal semen volume, 34.3% hypospermic and 2.8% hyperspermic. Table 4 summarizes the frequency percentage of microscopic semen profile in the study participants. Sperm concentration revealed 52.5% normal, 8.2% azoospermic, and 39.3% oligozoospermic males. Sperm morphology showed 57.7% teratozoospermic and 42.3% normal males. Sperm motility stood at 58.0% asthenozoospermia compared to 42.0% normal males. Leukospermia was observed in 58.5% participants relative to 41.5% normal males. Table 5 shows the association between socio-demographic characteristics and sperm concentration. There was no significant (p>0.05) relationship observed between socio-demographics and sperm concentration. The association of sexual history and co-morbidities with sperm concentration is presented in table 6. A significant association was observed between sperm concentration and erectile dysfunction (p=0.038), sperm concentration and STIs (p=0.026). Sexual history was an important factor as males with erectile dysfunction had a significantly high percentage (p=0.038, 80%) of abnormal sperm concentration compared to normal sperm concentration (20%). The percentage (63.6%) of males with a history of sexually transmitted infections and abnormal cell concentration was significantly (p=0.026) greater than those with normal cell concentration (36.4%).
|
|
|
|
|
|
|
4. Discussion
- The present study shows that, of the 600 males seeking for a remedy for the inability for their partners to conceive in our environment, 47.5% had abnormal sperm concentration and 52.5% had normal sperm concentration. Our finding is similar to some Nigerian studies conducted by Ajah et al, Owolabi et al, Green & Nwachukwu, Ugwa et al, Chika et al who respectively found a lower rate (39.9%, 31.8%, 48.4%, 47.6%, 46.0%) of male partners with abnormal sperm concentration compared to their normal counterparts [16-20]. The present study, however, is in contrast with the studies of Nanna & Unuajohwofia, Agu et al, Ugwuja et al, Abiose et al, Nwafia et al. who observed respectively a higher rate (53.0%, 53.6%, 74.0%, 60.3%, 56.4%) of Nigerian male partners with abnormal sperm concentration compared to their counterparts with normal sperm concentration [21-25]. Diallo et al. reported a 57.3% prevalence rate of abnormal sperm concentration in Senegalese men presenting with infertility [26].The present finding in a population of Nigerian men with abnormal sperm concentration is in line with several reports from other parts of the world (Spain, Scotland, France, Norway, Italy, Denmark, Belgium, Germany, Austria, Greece, Israel, Tunisia, China, and Canada) which attest that sperm concentrations of men from those countries have been diminishing over time [8]. In Nigeria, sperm concentration has been reported to be progressively declining [12] and this decline in semen concentration may be linked with environmental, nutritional, socio-economic or other factors [12-14]. Abnormalities in sperm concentration have been shown as the most common abnormalities and are typically a major contributory factor in men classified as infertile [27, 28]. The present study reports 67% primary fertility and 33% secondary infertility. Previous reports on national estimates of a total 5-year female infertility rate in sub-Saharan Africa show that secondary infertility (11-13%) compared to primary infertility (2-3%) at the global level predominates in Nigeria [4]. Various Nigerian studies on male infertility reports 83.7% [29], 75.7% (Lagos) [30], 73.0% (Ibadan) [31], 62.8% (Ilorin) [32], 70.0% (Ile-Ife) [17], 65.0% (Delta) [21], and 54.1% (Abakaliki) [23] secondary infertility. These reports contrast the findings of this present study, which calls for molecular studies in confirming and determining the cause of primary infertility in this region. However, similar findings were observed in some developing nations like Nigeria (65.0%), Senegal(57.4%), Sudan(62.4%) and Saudi Arabia(79.0%), where more than half of the study population were reported to have primary infertility [33, 26, 34, 35]. The pattern of seminal abnormality observed in this study ranging from the most prevalent to least was asthenozoospermia, teratozoospermia, oligozoospermia, and azoospermia. The commonest semen abnormalities observed from the pattern were asthenozoospermia and teratozoospermia. This is similar to the study of Nwafia et al who observed asthenozoospermia, and teratozoospermia as the commonest abnormality in a Nigerian population [25]. Ajah et al, Akinola et al, Agu et al, Adeniji et al observed asthenozoospermia as the commonest abnormality in Nigerian population studies [16, 30, 22, 31]. Asthenozoospermia was observed as the commonest abnormality in subfertile male Saudi, Los Angeles (USA), Indian population [36-38]. Teratozoospermia was the commonest semen abnormality found in the studies of Omokanye et al. (Nigeria), Enwuru et al.(Nigeria), Diallo et al.(Senegal), Husein et al.(Saudi Arabia), Elbardisi et al. (Saudi Arabia), Budni da Silva et al (Brazil) [32, 20, 26, 39-41]. In contrast with the present study, Peter et al, Green & Nwachukwu, Owolabi et al, reported oligozoospermia as the commonest semen abnormality in a Nigerian study [29, 18, 17]. In far contrast to this present study and many studies in Nigeria, Pethiyagoda (Sri-Lanka) observed azoospermia as the commonest abnormality in their study population [42]. The findings of this present study show that asthenozoospermia and teratozoospermia are the commonest semen abnormality in our environment. Poor motility and morphology are surrogate markers of poor sperm function [43]. Variations in the sample sizes and techniques of analysis in other studies compared may partly account for the differences in reported findings. These differences could be due to endocrine, ethnic, geographical, environmental, nutritional, or lifestyle variations. Specifically, the higher temperatures during most times of the year in Makurdi may affect sperm motility. In addition, genetic factors which are key to primary infertility may be a factor where differences are due to different polymorphisms in the genes involved in influencing these parameters.In analyzing the relationship of semen concentration with motility and morphology, a significant association was observed between these semen parameters. Among the male partners presenting with abnormal sperm concentration, 77.3% were asthenozoospermic and 77.5% teratozoospermic compared respectively to 22.7% and 22.5% observed in males with normal sperm concentration. In this study, most male partners of infertile couples produced normal volumes of sperm (62.8%), similar to reports of Butt et al, Nwafia et al, Ajah et al., Ugwa et al., Agu et al., Peter et al, Enwuru et al., Panti et al. [44, 25, 16, 19, 22, 29, 20, 45]. Strict adherence to informed standard semen collection criteria by the participants as well as the absence of retrograde ejaculation may have contributed to the normal semen volume observed. In this study, sperm concentration significantly associated with sperm volume where a majority of the male partners with low sperm concentration were hyperspermic (76.5%). The finding is similar to that of Maya et al., who found an inverse correlation of sperm concentration with sperm volume [46]. The relationship observed between abnormal sperm concentration and hyperspermia may be explained by the dilution effect of the ejaculate volume on sperm concentration. The present study observed 34.3% (206) hypospermic male partners with 54.4% (112) presenting with abnormal sperm concentration. The prevalence of hypospermia in fertile or infertile population is unknown. However, in a north Norwegian study involving 5739 infertile men, the proportion of hypospermic men had increased by 24.6% (p < 0.001) in the last decade (2003–2012) compared to the first decade (1993–2002) [47]. An adequate volume of ejaculate is required to carry spermatozoa into the female reproductive tract, and thus ejaculate volume is an important component of a semen analysis done to investigate male factor infertility. However, this parameter is often overlooked if other semen abnormalities are also present. Etiologies of hypospermia include artifactual, structural and functional factors. Artifactual factors encompass short abstinence period and incomplete collection, pathologic causes of low semen volume include retrograde ejaculation, failure of emission, ejaculatory duct obstruction, agenesis or aplasia of the seminal vesicles and prostate, seminal vesicle disease (infection, cysts), hypogonadism [48]. A high percentage of male partners with normal semen viscosity (85.8%) observed in the present study is similar to a Saudi Arabian (84.2%), Egyptian (75%) and Nigerian (64.7%) study in sub-fertile men with normal viscosity [36, 49, 20]. A significant seminal hyperviscosity (62.5%) was observed in men with abnormal sperm concentration in the present study. Gopalkrishnan et al., and Gonzales et al. showed that seminal hyperviscosity is associated with low sperm concentration [50, 51]. Mahrana & Salehb observed 25% seminal hyperviscosity in sub-fertile men with a further report of 37.5% leukocytospermia in sub-fertile men with seminal hyperviscosity [49]. The prevalence of semen hyperviscosity is estimated to be between 12-29% and could lead to male factor infertility [52, 53, 49]. The mechanisms through which hyperviscosity affect fertility are still unclear. However, semen hyperviscosity has been shown to contribute to male infertility through its association with changes in the chemical and physical features of semen that culminates into a negative impact on sperm motility and quality [51, 54]. Hypofunction of the prostate and seminal vesicles, as well as leukospermia and bacterispermia, are the major causes of semen hyperviscosity [55]. Mahrana & Salehb observed 37.5% leukocytospermia in sub-fertile men with seminal hyperviscosity [49]. Seminal hyperviscosity has been reported to be associated with leukocyte-derived reactive oxygen species resulting from male accessory gland infections [56, 57]. Other than leukocytospermia and genito-urinary tract infections, oxidative stress associated with varicocele, cigarette smoking, exposure to environmental pollution, idiopathic infertility may cause seminal hyperviscosity [55]. Sexually transmitted infections were reported in 7.3% of the study population. Among this group, a significant association of sperm concentration with a history of STI was observed. Male partners with low sperm concentration had a more prevalent history of STIs (63.6%) compared to male partners with normal sperm concentration (16.0%). Despite absent bacteriological examination in this present study; leukospermia was observed in 58.5% of the overall male partners with a significant association between semen concentration and leukospermia. Leukospermia in male partners with abnormal sperm concentration was 71.2% and those with normal sperm concentration 28.8%. There are similar reports of leukospermia in other studies [16, 20, 30, 31, 32, 58]. High leukocytes within the seminal fluid are an indicator of infection; this condition, marked by pus cells in the seminal fluid, is termed pyospermia [59]. Inflammation or infections in the male urogenital tract is accompanied by the infiltration of leukocytes into the semen [57]. Studies have shown a direct association between leukospermia and deterioration in semen parameters; sperm concentration, motility, morphology, and viability [60, 61]. To improve sperm quality, leukospermia need to be treated [62]. The pathophysiologic basis of the impact of leukocytes on semen parameters majorly lies in the ability of leukocytes to directly phagocytose spermatozoa and/or indirectly induce oxidative stress (due to overproduction of reactive oxygen species) on sperm cells [57]. Sperm elimination through phagocytosis can occur in human semen in isolated leukospermia which affects sperm quality. However, this effect is more aggressive in leukospermia coexisting with bacteriospermia [63]. The detrimental effects of reactive oxygen species (ROS) on sperm function include reduced sperm concentration, motility and normal morphology [64]. In addition to alteration in sperm characteristics, seminal ROS may interact with all sperm components, like polyunsaturated fatty acids present in large amounts in sperm membranes. Lipid peroxidation of sperm membrane marked by increased seminal malondialdehyde concentration levels has often been correlated with deterioration of sperm quality [65]. The process of lipid sperm membrane peroxidation is an aetiological factor of abnormal sperm function during leukospermia [57].While socio-demographic characteristics, HIV/AIDS, viral hepatitis, hypertension, and diabetes did not associate significantly with sperm concentration, abnormal sperm concentration was more in male partners that reported erectile dysfunction, suggestive of its correlation with infertility. The causes of this decrease in sperm quality in our environment have not been identified; however, environmental factors and lifestyle changes, as well as infections of the male reproductive, may play a role.
5. Conclusions
- Our study found abnormal semen parameters as a major contributor to male factor infertility in Makurdi, north-central Nigeria. This will provide a guide to intervention measures of improving fertility rates. Further scientific studies are needed in our environment to investigate the factors responsible for the high rate of primary male infertility and male factor infertility in general. This will aid professionals in presenting specific treatment modalities and options to the infertile couple for their informed decisions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML