-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2018; 8(4): 69-75
doi:10.5923/j.cmd.20180804.03

A Cost-Effective Panel Differentiating Neuroblastoma Infiltrating Bone Marrow from Acute Leukemia in Pediatric Patients
Nagwa Hassanein1, Faisal El Anzi2, Bargavi Balakrishnan3, Ali AlSaif3, Abdullah Al Khorasi3
1Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt, Clinical Pathology Consultant at Prince Faisal Cancer Center, KSA
2Pediatric Oncology Consultant at Prince Faisal Cancer Center, KSA
3Chemistry Specialist (Senior Technologist) at Prince Faisal Cancer Center, KSA
Correspondence to: Nagwa Hassanein, Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt, Clinical Pathology Consultant at Prince Faisal Cancer Center, KSA.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: The most common pediatric malignancy is acute leukemia mainly the lymphoblastic leukemia, Other malignancy characteristic to early childhood is neuroblastoma. Flow cytometry is an emerging tool for diagnosis of non-hematological malignancy which infiltrates bone marrow. Disseminated Neuroblastoma presenting with pancytopenia are morphologically indistinguishable from lymphoblast, this necessitates differentiating markers by flow-cytometry. Detailed comprehensive panels for acute leukemia and neuroblastoma diagnosis are established however not cost -effective. Aim of the study: Designing a short screening panel which can identify different type of Pediatric acute leukemia and discriminate neuroblastoma malignancy clearly and in economic way. Method: Bone Marrow aspirate from twenty neuroblastoma pediatric cases were collected for staging over a period of four years, from May 2013 to December 2017, in addition, ninety-five bone marrow aspirates from suspected pediatric acute leukemia cases were collected. All patients` bone marrow aspirates were extracted at Prince Faisal Cancer Center, Pediatric section and underwent screening for identification of phenotypic abnormal cells. The selected panel used included: CD45, CD3, CD4, CD8, CD19, CD10, CD20, Kappa/Lambda, CD34, CD117, CD15, CD33, CD38, CD71, CD61 and CD56. Acquisition was done on FACS CANTOII and FACS Diva software was used for analysis. Results: Out of twenty neuroblastoma staging cases, five uncover disseminated disease in the bone marrow aspirate which was successfully diagnosed by flow-cytometry using the previous combination of antibody. The most important markers were CD45 and CD56, The abnormal cells were positive for CD56 while negative for CD45 and other markers in the panel. One case was also positive for CD117. These findings were in agreement with immunohistochemistry markers as CD56, Synptophysin and Chromogranin applied on B.M biopsy and the original tumor biopsy. Out of the Ninety-Five pediatric acute leukemia cases’, eighty cases were diagnosed as acute lymphoblastic leukemia (ALL), B-cell type, eight cases were T-ALL while seven cases were acute myeloid leukemia (AML). Using the selected panel, the eighty B-ALL cases were accurately diagnosed; however, the T-All cases that were negative for surface CD3 needed extra cytoplasmic staining for cCD3 to finalize the diagnosis. Out of the seven AML cases, three were APML (AML-M3), Two were acute megakaryoblastic leukemia (AML-M7), one case was acute myelomonocytic leukemia (AML-M4) and one case acute myeloid leukemia with maturation (AML-M2). At the same time a validated comprehensive diagnostic panel was also used for all cases which include the essential markers for hematological diagnosis according to WHO classification of hematological malignancy. Conclusion: The limited screening panel selected has the capacity to categorize different acute leukemia subtypes, in addition to identifying neuroblastoma cells. The neuroblastoma promising markers were CD56 in association with CD45.
Keywords: Neuroblastoma, Flow cytometery, Acute leukemia
Cite this paper: Nagwa Hassanein, Faisal El Anzi, Bargavi Balakrishnan, Ali AlSaif, Abdullah Al Khorasi, A Cost-Effective Panel Differentiating Neuroblastoma Infiltrating Bone Marrow from Acute Leukemia in Pediatric Patients, Clinical Medicine and Diagnostics, Vol. 8 No. 4, 2018, pp. 69-75. doi: 10.5923/j.cmd.20180804.03.
Article Outline
1. Introduction
- Neuroblastoma prevalence is 12% out of total pediatric malignancy. It is heterogenous in presentation and nature. It is most common between the ages of 0 and 4 years and originates from the primordial neural crest cells which initiate the sympathetic neuronal ganglion and adrenal medulla. Dissemination of tumor cells to distant areas such as skin, liver and Bone marrow are the hallmark of high risk group in neuroblastoma [1]. Neuroblastoma presentation varies considerably based on the stage, while some present with asymptomatic localized abdominal mass, others may present with metastasis as first sign. 65% of cases present with suprarenal mass, while about 50% of patients have distant metastasis at presentation [2].Immunophenotypic identification of neuroblastoma has been highlighted by different studies. CD56, CD81 and Gangliosidase (GD2) were chosen as the best combination for neurobllastoma discrimination from other non hematopoietic cancer occur during childhood as Wilms’ tumor and germ cell tumor [3].Acute leukemia is the most frequently encountered Cancer with incidence of 35.3% in female and 34.8% in males aged 0-14 years in Saudi Arabia based on the cancer incidence report 2015. In comparison the adrenal tumors including neuroblastoma constitutes 3.1% in males and 4.9% in females for the same year [4].WHO classification for hematological malignancy has built the leukemia identification and categorization on Morphology, immunophenotyping, cytogenetic and molecular features. Also it establish The back bone Markers for phenotypic identification of different subtypes of acute leukemia. Phenotypic identification is considered the gold standard for leukemia categorization except for APML which is categorized cytogenetically. Different comprehensive panel proposed by different institutes either as a comprehensive panels from the beginning or as a screening panel followed by second lineage specified panel as Euro-flow, are applicable in diagnosis of acute leukemia. The cost of both strategy cause a burden on the limited resourced flow laboratory. (Arber et al, 2016).Although flow cytometry is an excellent quick tool for diagnosis of non- hematological malignancy, yet to be used in routine diagnosis, furthermore, the time needed for pathological preparation and immunohistochemistry procedures is extensive.The morphological similarity between neuroblastoma cells and lymphoblasts could be misleading in selecting the proper flow panel. Designing a cost effective short panel which cover both the acute leukemia and neuroblastoma includes CD56 in addition to lineage specific markers highlighted by WHO classification for hematological malignancies would be tremendously helpful to clear the diagnosis quickly and with affordable cost.
2. Subject and Methods
2.1. Subjects
- 20 cases of pediatric neuroblastoma and 95 cases of Pediatric Acute leukemia were collected over the period of four years, aged range between 1 month and 14 years old. The cases were admitted at the pediatric oncology department at Prince Faisal Cancer Center where they had bone marrow aspirate and biopsy for Neuroblastoma staging work-up. The cases collected between May 2013 and December 2017. Ethical committee approval was gained according to Helsinky declaration before starting the study. Informed consent from the pediatric guardian was gained as well.
2.2. Method
2.2.1. Flowcytometry Analysis
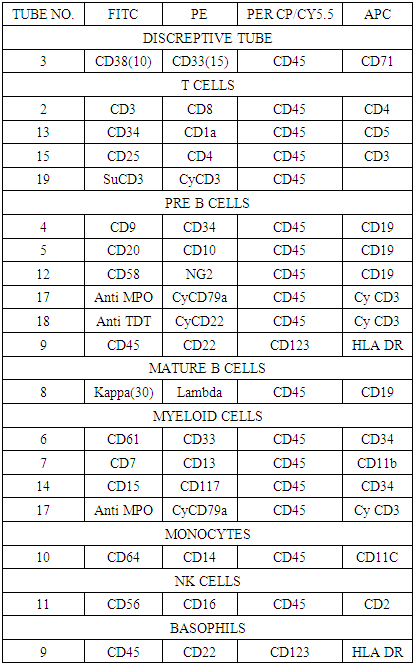
- Bone marrow aspirate samples anti-coagulated with sodium heparin were used. Clotted samples were rejected. Samples were processed within twenty –four hours of extraction. Samples were prepared gently using Centurion scientific K3 series centrifuge at 400g rpm speed. The fluorochrome combinations used in the panels are: fluorescein isothiocyanate (FITC), phycoerythrin (PE), Peridinin chlorophyll protein-Cy5.5 (PER-CP 5.5) and Allophycocyanin (APC). These fluorochromes have been selected for maximum resolution, stability, and ease of use from Becton Dicknson, USA. The combination specifically takes advantage of the availability of two lasers in modern flow cytometers, One blue and the other red laser.1X106 White Blood cells were added to each tube and incubated at room temperature (RT) in a dark area for 15-20mins. Red cells were lysed using 1x RBC lysing reagent (Becton Dickinson USA)followed by washing cells with cell wash buffer PH 7.0-7.4 (BD) with 1%BSA (bovine serum albumin) as a blocking agent to minimize non-specific binding. If needed, cells are incubated for 10 mins with 50ul of human IgG (immunoglobulin G) for blocking Fc-receptors on the cells. Finally cells are resuspended in a cell wash buffer with 1%bovine serum albumin (BSA) for acquisition in flowcytometer. Different combination of MoAb used are, CD38, CD3, CD20, CD15, CD61, anti-KAPPA were conjugated with FITC, CD33, CD10, CD8, CD56, anti-LAMBDA, CD117 were conjugated with PE, CD71, CD19, CD34, CD4 were conjugated with APC, and CD45 PER CP CY5.5 was used as a gating marker in all the tubes.Cases of suspected leukemia were stained by the regular comprehensive panel established and validated for the acute leukemia diagnosis (table 1).
|
3. Results
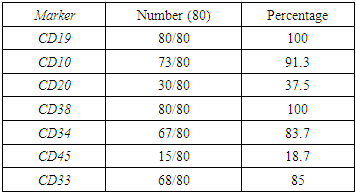
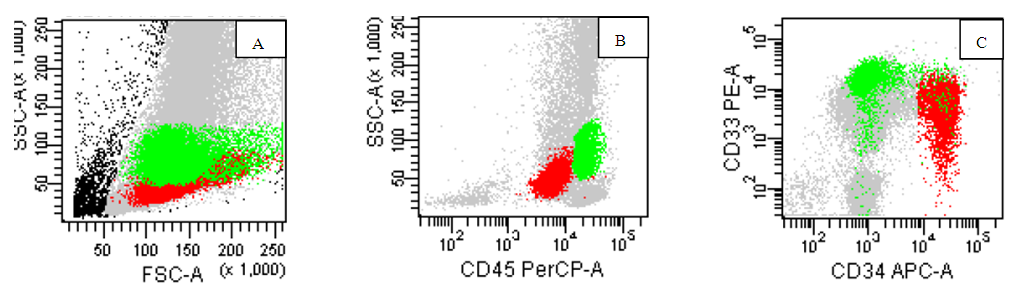
- Out of twenty pediatric cases screened for neuroblastoma staging, only five cases were proven positive for infiltration by flow cytometry and immunohistochemistry markers on biopsy.The identification of the neuroblastoma cells by flow was based on the location of malignant cells on CD45, side scatter histogram where the cells lie in the area of erythroid cells(negative for CD45 and has low side scatter proprieties). The cells were negative for B-cells markers, T-cell markers but positive for CD56 brightly. They were negative for CD38 to discriminate it from plasma cells. One case show positivity for CD117 in addition to CD56 while negative for CD34 as well as all other markers used in the panel. (Figure 1,2). On examination of Bone marrow biopsy, infiltration was evident with clustered collection of cells often surrounded by fibrosis. Synptophysin and chromogranin were stained on every biopsy for all cases with or without evidence by flow. Positive cases by flow show evidence of biopsy positivity by morphology and immunohistochemistry. Nineteen cases have abdominal mass resection preceded the staging workup with confirmed diagnosis by morphology as well as immunohistochemistry. In addition to a single case presented with pancytopenia for investigation which proven later to be disseminated neuroblastoma.Acute leukemia cases were categorized as eighty Precursor B ALL, eight Precursor T –ALL and seven AML. Among the 80 cases of B ALL, we had 7 Pro B-ALL, one mature B-ALL with positive surface expression of light chain, the other 72 cases are labeled as precursor B lymphoblastic leukemia. Further categorization was based on cytogenetic finding. B cells diagnosis was mainly based on invariable expression of CD19(80/80) 100%, CD10 (except infantile acute lymphoblastic leukemia (7/80) 8.7%, CD 20 in (30/80)37.5% of cases, lack of surface immunoglobulin except in the single mature B ALL 1/80, 1.2% case. While expressing aberrant CD33 in 12/80, 15% of cases, CD38 was expressed in (80/80)100% of cells. CD34 was negative in 13/80, 16.2%, CD45 expression 15/80 18.7% (figure 5) (table 2).
|
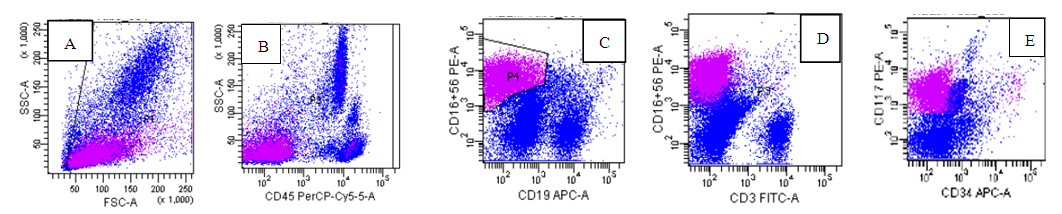 | Figure (1). The abnormal Neuroblastoma cells in Magenta are small in size(A), negative for CD45(B), CD3(D) and CD19(C) while positive for CD56(c) and CD117(E) |
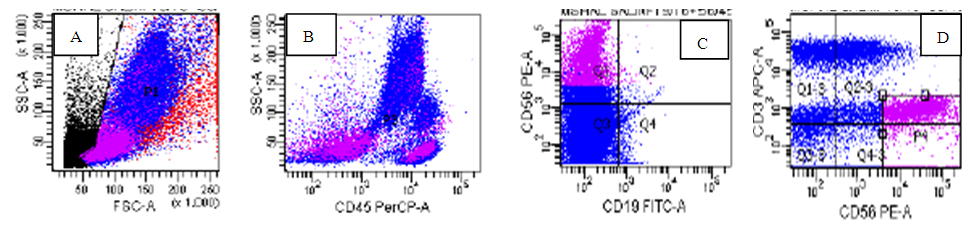 | Figure (2). Neuroblastoma case: The neuroblastoma cells in Magenta are positive only for CD56(C)(D). some NK cells are seen in the lymphocyte location on CD45, side scatter dot plot(B) |
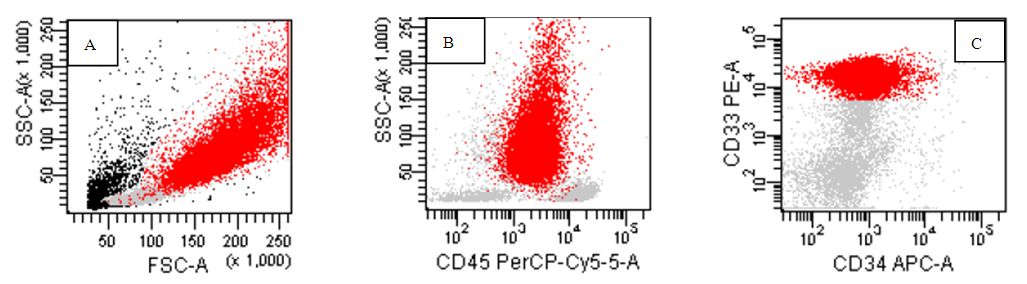 | Figure (4). AML-M3,hypergranular variant, the abnormal promyelocyte shown in red with high side scatter (B) and positive homogenous expression of CD33 while negative for CD34(C) |
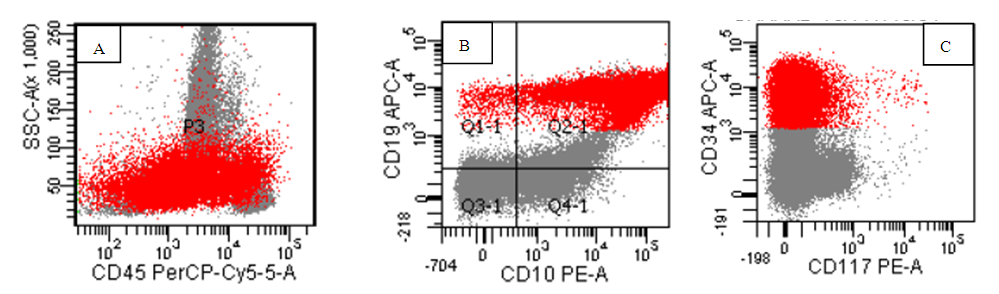 | Figure (5). A case of B –ALL, the blasts are red in color with heterogeneous expression for CD45 (A) positive expression of CD19, CD10(B), and CD34(C) |
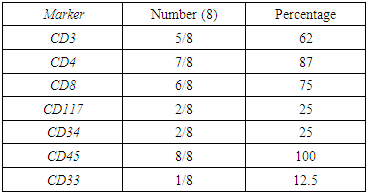
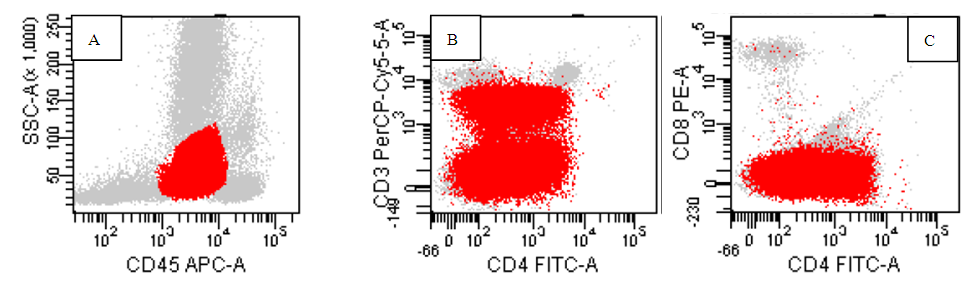 | Figure (6). A case of T ALL ,the population in red are blasts positive for CD45 (A) with partial positive for CD4, negative CD8 (C) while partial for surface CD3 expression(B) |
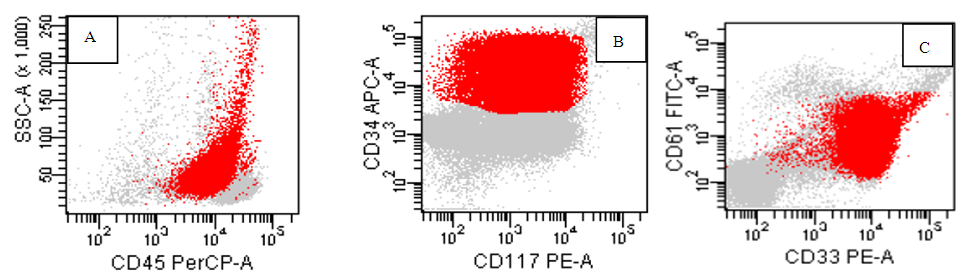 | Figure (7). A case of AML M7, the red blasts are positive for CD 45(A), CD34 and CD117(B), CD33 and CD61(C) |
|
4. Discussion
- The application of flow cytometry in the identification of non-hematological malignancies is an advancement in malignancy diagnosis. It helps in the identification of certain malignancies when time is extremely vital for proper patient care. The ability to discriminate neuroblastoma cells phenotypically will expedite the process of diagnosis in complex cases involving bone marrow when there are difficulties in accessing the original tumor. Although immunohistochemistry is the gold standard for diagnosis of neuroblastoma, it is costly and time consuming, flow-cytometry can however expedite the process of diagnosis [6].CD56 is a neuronal cell adhesion molecule (N-CAM) with known normal expression on Natural Killer (NK) cells, malignant plasma cells and other neuro-endocrinal tumors. The bright expression is a unique feature seen in neuroblastoma in comparison to non -hematological malignancy as Wilms’ tumor which express dimmer CD56 [7].Our flow cytometry panel was able to identify neuroblastoma cells disseminated to the bone marrow successfully based on the expression of CD56 only and lack of other hematological markers including CD45. The agreement observed between the flow cytometry and the gold standard morphology and immunohistochemistry highlight the promising role of flow in neuroblastoma detection in the tissue, this is in agreement with many study which highlight the bright expression of CD56 on neuroblastoma cells in comparison to other non hematological malignancy [8,9]. Although the combination of CD56, CD81, ganglioside (GD2) and neuron specific enolase (NSE) is used to discriminate the neuroblastoma cells [10], it is not cost effective for most of the labs in the developing country and it is not add an extra advantage over CD56 except if Wilms’ tumor is in the differential diagnosis. Some published study was investigating tissue neuroblastoma (suprarenal mass or abdominal mass and fine needle aspirate, our study along with other few studies focus was disseminated neuroblastoma cells in the bone marrow to help guide the cytometrist to discriminate neuroblastoma from leukemia cells which mimic it morphologically [11,12] and consider the possibility in the pediatric patient where both malignancy are common and this is in agreement with Chang et al and Leon et al who highlight neuroblastoma as the most common disseminated non hematological malignancy in pediatric patient. [13,14].Testing the expression of CD3, CD4, CD8, CD10, CD19, CD20, CD34, CD38, CD117, CD33 was helpful in discriminating acute leukemia and subtyping it. Immunohistochemistry staining concur the diagnostic finding of CD56 by showing the staining of synptophysin and chromogranin, however it is expensive and time consuming [15].Using CD45, side scatter dot plot, the location of the abnormal cells was a major key to identify the type of malignancy, neuroblastoma was invariably negative for CD45 with low side scatter while most of precursor B lymphoblastic leukemia had heterogenous pattern for CD45 starting from negative area and expand to the moderate positive area. While T ALL cells occupies the same location as myeloblasts and few number of B ALL cases, the pattern of marker expression was definite to distinguish them from each other [16].M3 was uniquely identified based on high side scatter with moderate CD45 expression, M4was a composite of a heterogeneous population which constituted a merge of myeloblast with immature monocytic cells which lies typically in the monocytic gate with bright CD45 and moderate side scatter presentation. M3 characterized by homogenous pattern of CD33 which is not seen in any type of AML or ALL as discussed by Goryza, W. [17]. While in M4, the monocytic population is brighter for CD33 in comparison to the myeloid population. Other types of AML expressed CD33in a heterogeneous pattern [18].We found the expression of CD19, CD10, CD20, a perfect combination to identify B cells in addition to K/L as declared by Campana and Pui. [19], While CD34, CD117, CD15 combination discriminate AML subtypes as M3, M2 and M4. M7 identification was built on expression of CD61 [20]. Based on WHO proposal for hematological malignancy, the expression of cMPO is inconsiderable in lymphoid leukemia except if the cells expressing myeloid markers strongly. We used this new add as a clue to omit cMPO from the screening of lymphoid neoplasm [5].For our T-ALL cases’ phenotype, the location of blasts on 45 side scatter dot plot cannot be discriminated from myeloblast, but the phenotype is clearly different by expression of Surface CD3 or /and either lacking CD4 or CD8, being double positive or double negative. Although the expression of CD7 is a constant finding in TALL cases, however it is not specific and not highlighted by WHO classification as a fundamental marker for T-ALL lineage specificity. In case of aberrant expression of myeloid markers as CD117, CD33 and CD13, the pattern was different in T ALL, it tend to be dimmer and partial in comparison of AML [21].Although Euro flow had already established a screening acute Leukemia orientation tube (ALOT) tube and lymphoma screening tube (LST), not many flow laboratory are able to use or even have access to get these tubes because of financial constraint [22].
5. Conclusions
- The selected screening panel have the potential to identify and subcategorize acute leukemia while discriminating neuroblastoma disseminated into the bone marrow with the acceptable sensitivity in comparison to detailed comprehensive panel usually used.
Disclosure
- Poster presentation have been published in ESSCA 2017 about using CD56 in the screening panel for neuroblastoma diagnosis. No conflict of interest among authers and no financial support provided.
ACKNOWLEDGEMENTS
- We thank Dr. Kehinde Oloko for reviewing and editing the manscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML