-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2018; 8(2): 26-32
doi:10.5923/j.cmd.20180802.02

Assessment of Blood Cotonine Level in Second Hand Smoke in Children and Its Relation to Respiratory Tract Infections
Nahla M. Salama1, Manal M. Zaher1, Rasha M. Gouda1, Aida A. Abd El-Hameed2
1Pediatric Department, Al-Azhar University, Faculty of Medicine for Girls, Egypt
2Clinical Pathology, Al-Azhar University, Faculty of Medicine for Girls, Egypt
Correspondence to: Aida A. Abd El-Hameed, Clinical Pathology, Al-Azhar University, Faculty of Medicine for Girls, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

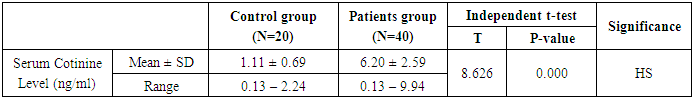
Background: Respiratory tract infections is the most common type of illness in children, in developing countries mortality due to acute lower respiratory tract infections is a significant problem, along with considerable morbidity and hospitalizations. Nicotine is one of the biomarkers specificto secondhand smoke exposure. Nicotine's major proximate metabolite is cotinine. On average, 75% of nicotine is converted to cotinine. Worldwide, 40% of children are regularly exposed to second hand smoke, parental smoking is considered to be a likely factor for respiratory illnesses in children. Objective: The study was designed to assess the blood Cotinine level in secondhand smoke children and its relation to respiratory tract infection. Method: serum level of cotinine was measured by enzyme linked immunosorbent assay (ELISA) in 40 patient with definite history of second hand smoke exposure with respiratory tract infection and 20 control group of apparently healthy children with matched age and sex. Results: There was a highly statistically significant increase in serum cotinine level in patients group compared to control group (p=0.000). There was a highly positive significant correlation between serum cotinine level and history of daily exposure to cigarette consumption with (p=0.000). There was a highly positive significant relation between serum cotinine level and number of smokers at home (p=0.010) and daily cigarettes grade (p=0.000). And also there was positive significant relation between serum cotinine level and difficulty of breathing (p=0.041). There was a highly statistically significant decrease in body weight, in body length and in birth weight (p=0.000), (p=0.000) and (P=0.019) respectively. Conclusion: The results of this study prove that the cotinine level was significantly higher in Children with respiratory tract infections and Children with lower respiratory tract infections had significantly higher serum levels of cotinine compared to children with upper respiratory tract infections. Growth parameters were significantly affected in children with respiratory tract infections and serum cotinine level was correlated to daily exposure to cigarettes consumption, cigarette grading and Number of smokers at home.
Keywords: Respiratory tract infections, Cotinine level, Secondhand smoke exposure(SHS)
Cite this paper: Nahla M. Salama, Manal M. Zaher, Rasha M. Gouda, Aida A. Abd El-Hameed, Assessment of Blood Cotonine Level in Second Hand Smoke in Children and Its Relation to Respiratory Tract Infections, Clinical Medicine and Diagnostics, Vol. 8 No. 2, 2018, pp. 26-32. doi: 10.5923/j.cmd.20180802.02.
1. Introduction
- Respiratory tract infections represent the most common type of illness in humans, and result in considerable morbidity, complications, and days lost from work and school [1]. In developing countries mortality due to acute lower respiratory tract infections is a significant problem, along with considerable morbidity and hospitalizations, particularly in children aged <5 years [2]. Environmental pollution, such as second hand smoke, defined as a mixture of exhaled main stream smoke from the smoker and side stream smoke from the burning end of a cigarette. Worldwide, 40% of children are regularly exposed to second hand smoke [3]. Environmental tobacco smoke especially parental smoking is considered to be a likely factor for respiratory illnesses in children [4]. There is a strong correlation between second hand smoke and respiratory symptoms [5]. Both passive and active tobacco smoking imposes very high risks for human health, the threat being inversely proportional to age, thus being most detrimental for small children if they become passive smoker [6]. It has been demonstrated that tobacco smoke toxic compounds really stimulate the activity of pro-inflammatory cytokines (IL-1, IL-6, IL-8, INFα and GM-CSF) and suppress the synthesis of anti-inflammatory cytokines, such as IL-10, while also affecting the subpopulation of T lymphocytes [8]. Cotinine is a metabolite of nicotine and one of the biomarkers specific to secondhand smoke exposure. On average, 75% of nicotine is converted to cotinine [7]. Cotinine’s half-life is about: 16 hours which is longer than nicotine’s (2h). Cotinine concentrations are more stable throughout the day, making it the preferred blood, saliva and urine biomarker [9]. Tobacco smoke toxic compounds increase the secretion of oxidative free radicals and decrease phagocytosis of granulocytes, thus being able to predispose the development of infectious diseases, especially bacterial [10].The aim of this study was to assess the blood cotinine level in second hand smoke children and its relation to respiratory tract infection.
2. Subjects and Methods
- This study was carried out on sixty children: forty children (Group 1) patient group with definite history of second hand smoke exposure, subdivided into: Group 1A (n=20): patients with upper respiratory tract infections (URTI) male represented 70% (n=14), while female represented 30% (n=6), and group 1B (n=20): patients with lower respiratory tract infection (LRTI) male represented 70% (n=14), while female represented 30% (n=6), compared to Group 2 (n=20): control group male represented 55% (n=11), while female represented 45% (n=9) of apparently healthy children with matched age and sex with group 1 selected from Al-Zahraa University Hospital, pediatric outpatients clinic. The study was conducted according to the rules of Ethics Committee by the Institutional Review Board (IRB) of Al-Azhar university and a written consent was obtained from children’s guardians. All cases and control groups were subjected to the following procedure1- Full history taking: With stress on age, sex, paternal and maternal smoking or history of any environmental exposure to second hand smoke from non-parental source, type of infant feeding, length of hospital stay.2- Complete clinical examination: Including Vital signs recording (body temperature, heart rate and respiratory rate), Weight, height and BMI, respiratory tract and systemic examinations.3- Laboratory investigations:A) Routine investigations: including Complete blood count (CBC): using Sysmex (Kx-21N) automated hematological counter.B) Specific investigations:- Determination of the serum level of cotinine by enzyme linked immunosorbent assay (ELISA) technique using the ElabscienceInc kit, Catalog No: E-EL-0064.1) Sampling5ml morning venous blood sample was collected under complete aseptic conditions from the 60 children were divided as followsA- Tow ml injected into EDTA tube for CBC, B- 3ml without anticoagulant was allowed to clot for 30 minutes, the serum was separated by centrifugation for 10 minutes at approximately 3000 g at room temperature then was preserved and was freezed in one E pendorf tube at -20°C until the cotonine assay.Principle of the testThis ELISA kit uses Competitive-ELISA as the method. The microtiter plate provided in this kit was pre-coated with Cotinine. During the reaction, Cotinine in the sample or standard competes with a fixed amount of Cotinine on the solid phase supporter for sites on the Biotinylated Detection Ab specific to Cotinine. Excess conjugate and unbound sample or standard was washed from the plate, and Avidin was conjugated to Horseradish Peroxidase (HRP) which was added to each microplate well and was incubated. Then a TMB substrate solution was added to each well. The enzyme-substrate reaction was terminated by the addition of a sulphuric acid solution and the color change was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of Cotinine in the samples was determined by comparing the OD of the samples to the standard curve.Detection Range: 0.625-40ng/mLSensitivity:The minimum detectable dose of Cotinine is 0.375ng/mL (The sensitivity of this assay, or lowest detectable limit (LDL) was defined as the lowest protein concentration that could be differentiated from zero).Specificity: This kit recognizes natural and recombinant Cotinine. No significant cross-reactivity or interference between Cotinine and analogues was observed.4- Statistical analysis:Data were collected, coded, revised and entered to the Statistical Package for Social Science (IBM SPSS) version 20. The data were presented as number and percentages for the qualitative data, mean, standard deviations and ranges for the quantitative data with parametric distribution.Z-score of anthropometric measurement was calculated according to the following formula: Chi-square test was used in the comparison between two groups with qualitative data and Fisher exact test was used instead of the Chi-square test when the expected count in any cell found less than 5.
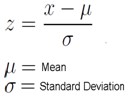 The comparison between two independent groups with quantitative data and parametric distribution were done by using Independent t-test. While the comparison between more than two groups with quantitative data and parametric distribution were done by using One Way ANOVA followed by post hoc analysis using Tukey's test. Spearman correlation coefficients were used to assess the relation between two quantitative parameters in the same group. Receiver operating characteristic curve (ROC) was used to assess the best cut off point with its sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following: P > 0.05: Non significant (NS), P < 0.05: Significant (S)P < 0.01: Highly significant (HS).
The comparison between two independent groups with quantitative data and parametric distribution were done by using Independent t-test. While the comparison between more than two groups with quantitative data and parametric distribution were done by using One Way ANOVA followed by post hoc analysis using Tukey's test. Spearman correlation coefficients were used to assess the relation between two quantitative parameters in the same group. Receiver operating characteristic curve (ROC) was used to assess the best cut off point with its sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following: P > 0.05: Non significant (NS), P < 0.05: Significant (S)P < 0.01: Highly significant (HS).3. Results
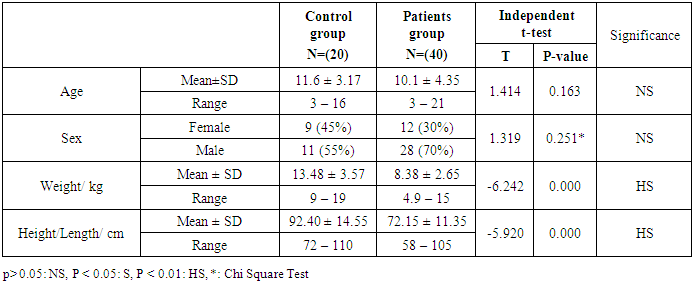
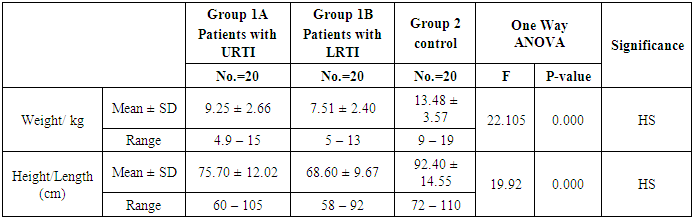
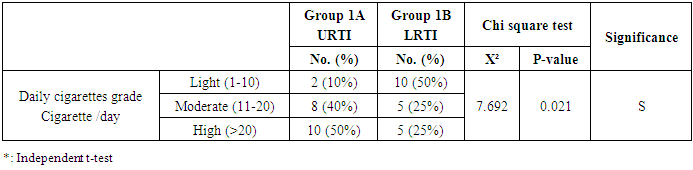
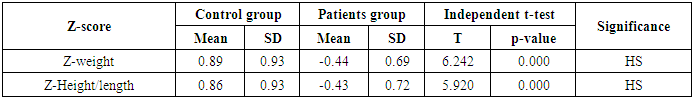
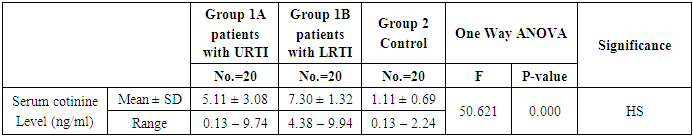
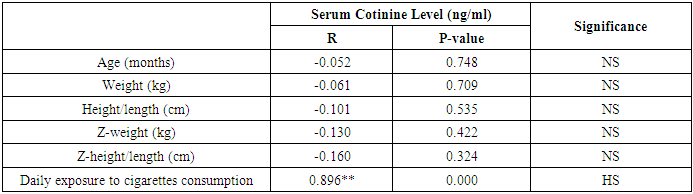
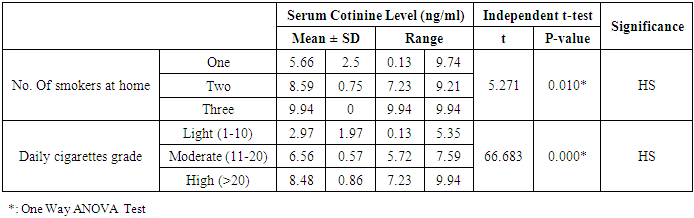
- This study was performed on sixty children: forty children patient group with definite history of second hand smoke exposure, male represented 70% (n=28), while female represented 30% (n=12) compared to twenty (n=20) children control group male represented 55% (n=11), while female represented 45% (n=9) of apparently healthy children with matched age and sex. There was highly statistically significant decrease in body weight and length in patients group when compared to control group (P<0.01) as shown in table (1). There was statistically significant decrease in body weight and height among patient groups URTI (G1A) and LRTI(G1B) when compared to control group (P<0.01) as shown in table (2). There was statistically significant difference in daily cigarettes grade in group 1B (LRTI) when compared to group 1A (URTI) as shown in table (3). There was highly statistically significant decrease in body weight and height regarding Z-score among patient group when compared to control group (p<0.01) as shown in table (4). There was highly statistically significant increase in serum cotinine level in patients group when compared to control group as shown in table (5). There was highly statistically significant increase in serum cotinine level among patients with URTI and patients with LRTI when compared to control group as shown in table (6). There was highly positive significant correlation between serum cotinine level and history of daily exposure to cigarette consumption as shown in table (7) & Fig (1). There was highly positive significant relation between serum cotinine level and number of smokers at home and daily cigarettes grade as shown in table (8) Fig (2).
|
|
|
|
|
|
|
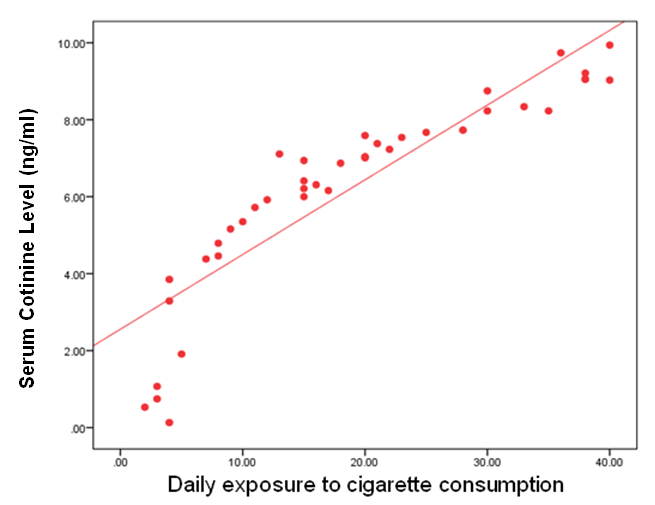 | Figure (1). Correlation between serum cotinine level and daily exposure to cigarette consumption in all patients groups |
|
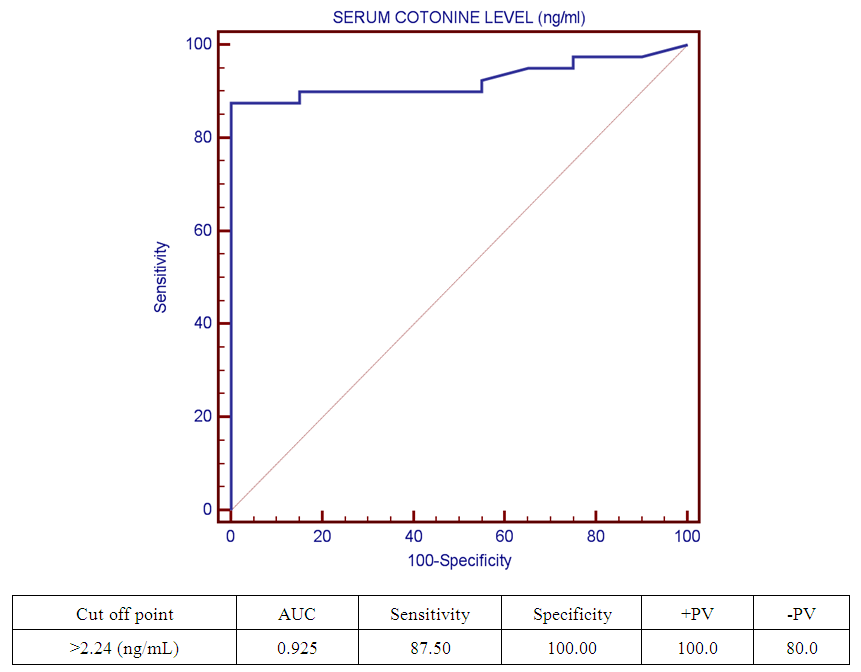 | Figure (2). Receiver operating characteristic curve (ROC) curve show cut off point of serum cotinine level among studied groups |
4. Discussion
- Respiratory tract infections represent the most common type of illness in humans, and result in considerable morbidity, complications, and days lost from work and school [1]. Worldwide, an estimated 603,000 deaths were attributed to SHS in 2004, which was approximately 1.0% of worldwide mortality. (165,000) of these deaths were from lower respiratory tract infections [3]. There is a strong correlation between second hand smoke and respiratory symptoms [11]. The study was designed to assess the blood Cotinine level in secondhand smoke children and its relation to respiratory tract infection.Our study was conducted on sixty children: forty children (Group 1) patient group with definite history of second hand smoke exposure, subdivided into:Group 1A (n=20): patients with upper respiratory tract infections (URTI) male represented 70% (n=14), while female represented 30% (n=6), and group 1B (n=20): patients with lower respiratory tract infection (LRTI) male represented 70% (n=14), while female represented 30% (n=6), compared to Group 2 (n=20): control group male represented 55% (n=11), while female represented 45% (n=9) of apparently healthy children with matched age and sex with group 1.All cases and control groups were subjected to full history taking with stress on age, sex, paternal and maternal smoking or history of any environmental exposure to second hand smoke from non-parental source, type of infant feeding. Clinical assessment of respiratory infections, physical examination (both general and systemic examination particularly chest) and laboratory investigations including general and specific in the form of serum cotinine level estimation.The impact of infections on growth has been well documented. Lower respiratory tract infections reduce weight gain in young children by 14.7 g/d of infection [12].Among the study results of patients groups exposed to second hand smoke, there was highly statistically significant decrease in body weight when compared to control group = 8.38 ± 2.65(4.9 – 15), 13.48 ± 3.57(9 – 19) p=0.000 (table 12) in both groups respectively, which agree with [13], who found that eight biological, early life and social factors impacted on rapid weight loss. Smoking emerged as highly important factor. Postnatal exposure through smoking in the same room as the child was independently associated with an increased risk of more rapid weight loss. Among this study, there was a highly statistically significant decrease in z-height in patients groups who had definite history of exposure to second hand smoke which it matched with [14]. The results of the study indicate that exposure to prenatal and postnatal smoking had a persistent negative effect on height until adolescence; children who were exposed in these periods were shorter since birth until adolescence compared with those who were not exposed. But [15] found no effect of household smoke exposure or interactions involving exposure to household smoking on height. Also [16], who found no significant differences between the cotinine-based group and the control group with regard to body height. And [17] found that the evidence is inadequate to infer the presence or absence of a causal relationship between exposure to secondhand smoke and children’s height.As regard the number of cigarette smoked per day by care givers and daily cigarette grade and its relation to serum cotinine level in the studied children we found highly significant correlation between them, that is supported with [18] who found that the cotinine levels of children living with smoker (s) increase in direct proportion to the intensity of the smoking habits of the cohabitant smokers; this finding is especially significant among children who live with smokers who consume a high number of cigarettes daily (C20). This significant relationship shows that heavy smokers, in addition to risking adverse effects on their own health, are endangering people living in the same environment.[19] a caregiver who smokes a lot of cigarettes every day at home. Consuming >20 cigarettes/day was found to be significant predictive factor of higher cotinine levels in children. However, there is no risk-free level of exposure in environmental tobacco smoke, more smoking by caregivers lead to higher cotinine levels of children.As regard the number of smokers at home and serum cotinine level we found highly statistically relation. This is in mismatch with [18] who found that number of smokers at home did not have significant effects on cotinine level.Regard the level of cotinine among studied patients groups there was a highly statistically increase in patients group when compared to control group mean level was: 6.20 ± 2.59 (0.13 – 9.94), 1.11 ± 0.69 (0.13 – 2.24) ng/ml respectively (table 5) and by using Tukey's test, there was highly statistically significant decrease in patients group with LRTI when compared to patients with URTI and control group (table 6). This was in agreement with [19] who found that SHS exposure is an established risk factor for infant LRTI severity, and that infants with history of SHS exposure were associated with a longer length of hospital stay for LRTI, which was not the case in those without this history. And also with [20] in the study, childhood exposure to SHS was associated with a higher period prevalence of hospital admission for pneumonia than those not exposed. In a previous study by [21] they highlighted the role of the environmental tobacco smoking in the development of severe pneumonia in under-five Years children. [22] also confirms that exposure to all types of passive smoke, in particular maternal smoking, causes a statistically significant increase in the risk of infants developing lower respiratory infections in the first two years of life.[17] found that passive smoking was recognized as a cause of lower respiratory infection in children. [3] found that worldwide an estimated 603,000 deaths were attributed to SHS in 2004, which was approximately 1.0% of worldwide mortality. (165,000 deaths) of these deaths were from lower respiratory tract infections.Cut off point of cotinine level in the serum of children exposed to second hand smoke was 2.24 ng/ml with high sensitivity and specificity (87.5%, 100% respectively) which signify that it is sensitive and specific marker in evaluation of SHS metabolites in second hand smoke children.
5. Conclusions
- Our findings from current study revealed that Children with respiratory tract infections had significantly higher serum levels of cotinine. Children with lower respiratory tract infections had significantly higher serum levels of cotinine compared to children with upper respiratory tract infections. Growth parameters were significantly affected in children with respiratory tract infections. Serum cotinine level is correlated to daily exposure to cigarettes consumption, cigarette grading and Number of smokers at home.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML