-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2018; 8(2): 21-25
doi:10.5923/j.cmd.20180802.01

Estimation the Serum Levels of Endothelin-1, and Nitric Oxide and Its Relation to Occurrence of Intradialytic Hypertension among Individuals Receiving Maintenance Hemodialysis
Osama Mohamad Ahmad 1, Ahmed Salama Al Adl 1, Mahmoud Saad Berengy 1, Mahmoud Hussein Sayed 2
1Internal Medicine Department, Al-Azhar University Faculty of Medicine Damietta, Egypt
2Clinical Pathology Department, Al-Azhar University Faculty of Medicine Damietta, Egypt
Correspondence to: Ahmed Salama Al Adl , Internal Medicine Department, Al-Azhar University Faculty of Medicine Damietta, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

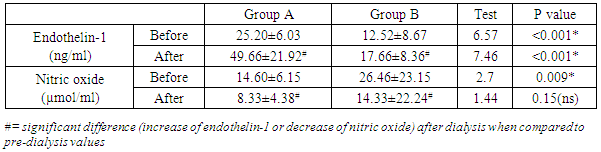
Background: Intradialytic hypertension is a common problem observed in patients on maintenance hemodialysis and considered as an independent predictor of adverse clinical outcomes. Aim of the work: estimation the serum levels of endothelin-1, and nitric oxide and its relation to occurrence of intradialytic hypertension in patients on maintenance hemodialysis. Methodology: the study is a case control study included 60 patients with end stage chronic renal disease (ESRD) on maintenance hemodialysis, selected from Nephrology Units of Al-Azhar University hospital New Damietta, Kafr Saad and Elzarka General Hospitals during the period from Feb. 2015 to March 2017. They were divided into two groups according to the presence of intradialytic hypertension. Group A: 30 patients with intradialytic hypertension (IHD). Group B: 30 patients without intradialytic hypertension (IHD), All patients were submitted to full history taking and clinical examination; laboratory investigations included routine investigations and estimation of endothelin-1 and nitric oxide before and after dialysis. Results: the serum level of endothelin-1 before dialysis ranged from 2.3 (ng/ml) to 42 (ng/ml) and there was statistically significant increased in group A in comparison to group B and After dialysis, there was statistically significant increased (49.66±21.92) in group A in comparison to group B (17.66±8.36). Before dialysis, nitric oxide ranged from 5.77(µmol/ml) to 95.60(µmol/ml) with a mean of 20.53±17.83 with statistically significant decreased (14.60±6.15) in group A in comparison to group B (26.46±23.15). On the other hand, after dialysis, it ranged from 3.02 to 91.07 with a mean of 11.33±16.18 and there was statistically non significant difference between both groups. Conclusion: Intradialytic hemodynamic changes is a complex interplay of endothelin-1, and endothelial function. endothelin-1 and nitric oxide are strongly involved in the occurrence of intradialytic hypertension Among Individuals Receiving Maintenance Hemodialysis.
Keywords: Endothelin-1, Nitric oxide, Intradialytic hypertension
Cite this paper: Osama Mohamad Ahmad , Ahmed Salama Al Adl , Mahmoud Saad Berengy , Mahmoud Hussein Sayed , Estimation the Serum Levels of Endothelin-1, and Nitric Oxide and Its Relation to Occurrence of Intradialytic Hypertension among Individuals Receiving Maintenance Hemodialysis, Clinical Medicine and Diagnostics, Vol. 8 No. 2, 2018, pp. 21-25. doi: 10.5923/j.cmd.20180802.01.
Article Outline
1. Introduction
- Intradialytic hypertension, defined as an increase in systemic arterial blood pressure during hemodialysis (HD), and observed in about 15% of the maintenance HD population [1]. Intradialytic hypertension has recognized as an independent predictor of adverse clinical outcomes, including non–access related hospitalization and mortality [2]. Acute hemodynamic changes are still the most frequent complications during hemodialysis (HD) treatment. This causes discomfort, reduces treatment efficacy, and thus increases long-term cardiovascular problems and consequently the morbidity of these patients. This also contributes to increased monitoring and workload of nurses and physicians, thereby increasing treatment costs [3]. Despite its clinical significance, little is known about the patho-physiologic mechanisms underlying intradialytic hypertension. Moreover, a causal relationship between intradialytic hypertension and adverse clinical outcomes has yet to be established [1]. Endothelin-1 (ET-1), a vasoactive peptide, is believed to contribute to the pathogenesis of vascular abnormalities, such as hypertension and atherosclerosis. ET-1 elicits its biological effects through the activation of two receptor subtypes: ET-A and ET-B that belong to a large family of transmembrane guanine nucleotide-binding protein-coupled receptors [4]. Nitric oxide is a highly active small molecule that participates in many physiologic processes, among them vasodilatation, neurotransmission and host defense. Endothelial dysfunction, which is characterized by impairment of nitric oxide bioavailability, is an important risk factor for both hypertension and cardiovascular diseases [5].
2. Aim of the Work
- Estimation the serum levels of endothelin-1, and nitric oxide and its relation to occurrence of intradialytic hypertension in patients on maintenance hemodialysis.
3. Patients and Methods
3.1. Study Design
- The present study is a case control study included 60 patients with end stage chronic kidney disease on maintenance hemodialysis 3 sessions per week, were divided into two groups according to presence of intradialytic hypertension to Group A: thirty (30) patients with intradialytic hypertension (IHD) 15 males and 15 females. Group B: thirty (30) patients without intradialytic hypertension (IHD) (18 males and 12 females). Both groups were selected from Nephrology Units of Al-Azhar University hospital New Damietta, Kafr Saad and Elzarka General Hospital, Damietta, Egypt During the period from Feb. 2015 to March 2017.
3.2. Study Protocol
- All patients were subjected to the history taking and clinical examination. Assessment of the following parameters: body weight before and after dialysis, blood pressure, before the session directly, during (every hour) and after hemodialysis by 1 hour. In addition, the following laboratory investigations were done: endothelin -1, nitric oxide before and after hemodialysis, Endothelin -1 was estimated by DRG® Endothelin-1 (Human, Rat, Mouse, Porcine, Bovine, Canine) ELISA (EIA-3420) Kit. This Enzyme Immunoassay kit is designed to detect a specific peptide and its related peptides based on the principle of “competitive” enzyme immunoassay. Also blood urea, serum uric acid, albumin, CBC (hematocrit value), serum Na, K and Calcium were estimated before the hemodialysis session.Blood access was arterio-venous fistula. Dialysis was performed for 4 hours, three times weekly using conventional heparin, blood flow rate was 300–350ml/min with a dialysate flow rate of 500 ml/min. Ultrafiltration varied according to patient’s actual weight. The membrane used was polysalphone with surface area suitable for each patient. Bicarbonate was the buffer used throughout the study for all patients.
3.3. Inclusion Criteria
- Patients with end stage chronic kidney disease on regular hemodialysis, 30 patients with intraialytic hypertension and 30 patients without intraialytic hypertension.
3.4. Exclusion Criteria Were
- Patients with duration less than six months on regular hemodialysis, diabetic patients, patients with previous cardiovascular disease, uncontrolled hypertension, recent major cardiovascular surgery, connective tissue disease as a cause of CRF, chronic liver disease.
3.5. Ethical Aspects
- The informed consent was obtained from all participants. The research protocol did not interfere with any medical recommendations or prescriptions.
3.6. Statistical Analysis of Data
- The collected data was organized, tabulated and statistically analyzed using Statistical Package for Social Science (SPSS) version 17 (SPSS Inc, Illinois, Chicago, USA). For quantitative data, mean, standard deviation (SD), minimum and maximum were calculated and for comparison between two groups, the students (t) test was used. For comparison between the same group at two different points of time (i.e. Before and after dialysis), paired samples (t) test was used. For qualitative (categorical data), frequency and percent distribution were calculated, and for comparison between groups, the Chi square (x2) was calculated. For interpretation of results, p value less than or equal to 0.05 was considered significant.
4. Results
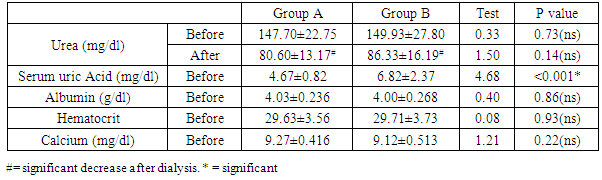
- In the present work, age ranged from 19 to 64 years with a mean of 45.10±11.65 years. 33 (55%) of study patients were males and 27 (45.0%) were females with male: female ratio of 1.22: 1. Weight before dialysis ranged from 54 to 89 kg with mean of 73.63±7.24 kg; while after dialysis, it ranged from 50 to 87 kg, with a mean of 71.98±6.51 kg, and there was statistically insignificant difference between group A and group B either before or after dialysis. (Table 1).
|
|
5. Discussion
- In the present study, there was statistically significant increase in hematocrit after dialysis in comparison to values before dialysis in groups A & B. Similar results were reported by [6]. As regard serum calcium before dialysis, it ranged from 8.30± to 10.10 with a mean of 9.19±0.46, and was statistically non significant difference between group A and group B These results are in contradiction to [6]. who reported that, there was statistically significant increase of calcium in IDH and proposed that, it may work a minimal part in Pathophysiology of IDH. The possible explanation may be attributed to dialysate buffer. During HD, in response to mechanical and hormonal stimuli, endothelial cells synthesize and release humoral factors, including the endothelial-derived relaxing factor, NO, and the vasoconstrictive factor, ET-I. It has been shown that the NO and ET-I balance is involved in the pathogenesis of intradialytic hypertension [7].Teng et al. 2015 [6] reported that, during HD there are usually two physical processes occurring, diffusion and ultrafiltration, which result in a reduction in circulating plasma volume. To maintain adequate blood pressure, the usual response is an increase in cardiac output and peripheral vascular resistance. The primary mechanism is the acute stimulation of the sympathetic nervous system, with an increase in stroke volume and heart rate, and vasoconstriction with an increase in peripheral vascular resistance. El-Shafey et al. 2008 [6] reported that, the intradialytic changes of the endothelium-derived molecules (NO and ET-1) can be explained by four assumptions: first, simple accumulation (activation exceeds removal by dialysis and vice versa); second, by a possible increased secretion and stimulation that are mostly related to an endotoxin that crosses the dialysis membrane; third, by lack of inhibition that may play a role (asymmetric dimethylarginine is the main endogenous inhibitor of NO). In addition, membrane-blood interaction leads to activation of monocytes with increased production of cytokines [mainly interleukin-1 (IL-1) and tumor necrosis factor–α (TNF-α)]. IL-1 and TNF-α are chronically elevated in HD patients, and IL-1 increases further during HD.Teng et al. (2015) [6] found that the serum levels of NO were significantly decreased in hemodialysis patients with IDH, and the plasma levels of ET-1 were significant elevated IDH. Thus, the NO/ET-1 balance was significantly depressed in IDH compared the other patients, which may be the cause of the inappropriate elevation of peripheral vascular resistance. However, the possibility of production of other vasoconstrictions or removal of other vasodilators during HD still cannot be excluded completely.In the present study, there was statistically significant increase of endothelin 1 in group A in comparison to group B before and after dialysis. In addition, there was statistically significant increase in endothelin-1 after dialysis in comparison to their values before dialysis in groups A & B. On the other hand, there was statistically significant decrease of NO in group A in comparison to group B before and after dialysis. Furthermore, there was statistically significant decrease of nitric oxide after dialysis in comparison to their values before dialysis in groups A and B. There are discordant data about the ET-1 levels in HD patients since it has been reported to be unchanged [9] while Sheen et al., 2007 [10] reported that there is increase in ET-1 in HD patients, but Odetti et al., 2006 [11]. reported that there is decreased in ET-1 in HD patients post-dialysis. Furthermore, it was reported that ET-1 levels vary according to type of membrane used during HD and UF rate [12]. Our results are in agreement with Safa et al. (2014) [13]. who demonstrated increased plasma ET-1 levels in hypertensive ESRD patients during HD possibly stimulated by volume depletion with sympathetic activation, which may attenuate hypertensive HD effects, thus contributing to intradialytic and interdialytic hypertension.The results of the present study are in agreement with Assimon et al. 2017 [14]. who reported that, maintenance HD patients with intradialytic hypertension have abnormal in vivo endothelial cell function. They observed a 50% difference in the number of endothelial progenitor cells in those with intradialytic hypertension as compared other HD patients without IDH. In addition, there was impaired endothelial-dependent vasodilation among those with intradialytic hypertension. In addition, Abramoff et al. (2015) [15] reported that ET-1 plasma levels have been found to be higher in HD hypertensive patients compared to other HD normotensive subjects.Teng et al. (2015) [6] comparing patients with intradialytic increases of BP to patients without IDH, hypertensive-prone patients exhibited an increase in systemic vascular resistance and a significant decrease in nitric oxide relative to endothelin-1 at the end of dialysis. In a smaller study by Treweeke et al. (2017) [7] on nine patients with intradialytic increases in mean arterial BP, endothelin-1 significantly increased during HD. and, these study suggested that intradialytic hypertension mediated by an imbalance in important endothelial-derived vasoregulators.
6. Conclusions
- Intradialytic hemodynamic changes is a complex interplay of endothelin-1, and endothelial function. endothelin-1 and nitric oxide are strongly involved in the occurrence of intradialytic hypertension Among Individuals Receiving Maintenance Hemodialysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML