-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2018; 8(1): 7-13
doi:10.5923/j.cmd.20180801.02

Polymorphism of Interlukin-12B Gene and Serum Level of Interlukin-12p70 in Rheumatoid Arthritis Egyptian Patients
Entsar R. Mokhtar1, Asmaa S. Hassan1, Eman F. Mohamed2, Doaa M. Zakria2, Aml E. Abdou3, Marwa M. Abdel Rahim4
1Department of Clinical Pathology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
2Department of Internal Medicine, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
3Department of Microbiology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
4Department of Physical Medicine, Rheumatology and Rehabilitation, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Entsar R. Mokhtar, Department of Clinical Pathology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

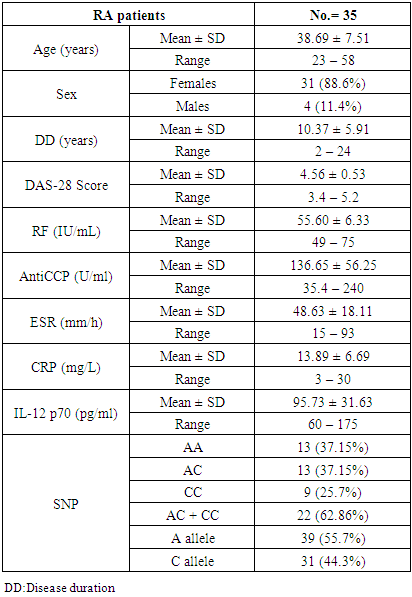
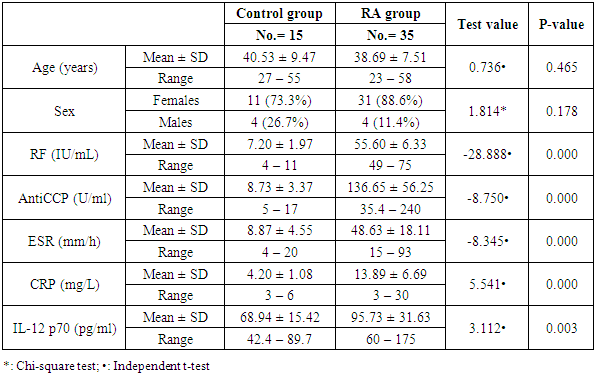
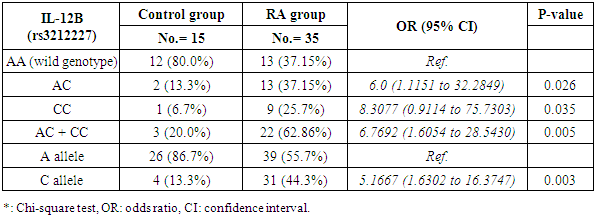
Background: Rheumatoid arthritis (RA) is a common autoimmune disease characterized by synovial infiltrates and progressive cell-mediated destruction of the joints. Several studies reported that genes encoding cytokines, which regulate CD4+ T cell differentiation, are attractive genetic factors that may predispose to susceptibility and severity of RA. Interleukin-12 (IL-12) plays a critical role in the pathogenesis of T cell-mediated autoimmune disease as RA. Objective: to investigate single nucleotide polymorphism (SNP) (rs3212227 (A/C) of the IL-12B gene and serum level of IL-12 P70 in RA patients trying to find their relation to susceptibility and severity of the disease. Methods: Thirty five patients with RA and fifteen healthy subjects were examined for IL-12B gene polymorphisms using polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method. Serum level of IL-12p70 was measured by ELISA. Results: The frequency of IL-12B (rs3212227) AC, CC, AC+CC genotypes were significantly higher in RA patients as compared with healthy subjects (P = 0.026; P = 0.035, P = 0.005) respectively. The frequency of (rs3212227) C allele also showed similar result (P = 0.003). IL-12B (rs3212227) AC+CC genotypes were associated with exacerbation of RA. Serum level of IL-12p70 was significantly higher in RA patients as compared with healthy subjects (P = 0.0001). Significant positive correlation was found between IL-12p70 serum level and DAS-28 score, Anti–CCP, RF. Conclusion: Present findings suggested that IL-12B (rs3212227A/C) polymorphism was associated with RA susceptibility and severity and IL-12p70 serum levels can reflect RA disease activity.
Keywords: Rheumatoid arthritis, Interleukin-12B gene, IL-12 P70, Single nucleotide polymorphism
Cite this paper: Entsar R. Mokhtar, Asmaa S. Hassan, Eman F. Mohamed, Doaa M. Zakria, Aml E. Abdou, Marwa M. Abdel Rahim, Polymorphism of Interlukin-12B Gene and Serum Level of Interlukin-12p70 in Rheumatoid Arthritis Egyptian Patients, Clinical Medicine and Diagnostics, Vol. 8 No. 1, 2018, pp. 7-13. doi: 10.5923/j.cmd.20180801.02.
Article Outline
1. Introduction
- Rheumatoid arthritis is a common autoimmune disease characterized by the proliferation of synovium and the infiltration of chronic inflammatory cells, it results in significant disability and early mortality [1]. Cytokines from synovium and inflammatory cells are thought to be important in the initiation and perpetuation of RA [2]. Cytokines have crucial roles in the regulation of inflammatory and immune responses, and polymorphisms of the cytokine genes may result in functional change in the proteins or their expression, which furtherly lead to modified immune response [3]. Interlukin-12 is one of the heterodimeric cytokines, which composed of two disulphide-linked subunits, of molecular weights of 40 kDa heavy chain; p40 (encoded by IL-12B gene), and 35 kDa light chain; p35 (encoded by IL-12A gene), neither of which has been found to display any significant biological function alone. Instead, a heterodimeric form of IL-12p70, mediates a biological response, whereas a p40 homodimer acts as an IL-12 antagonist [4], because p40 subunit may form homodimers, which can bind to IL-12 receptors and inhibit the activation induced by the IL-12 heterodimer as well as modulate IL-12 function [5, 6]. IL-12 is a pro-inflammatory cytokine produced by cells of the innate immune system as well as by B cells. It is considered as a connecting point for innate and adaptive immunity [7]. IL-12 regulates the balance between Th1andTh2 cells, as well as it enhances cytotoxic T cell-mediated lysis and natural killer (NK) cell activity [8]. In addition, it plays a central role in promoting the differentiation of naive CD4+ T cells into mature interferon-(IFN-γ) producing Th1effector cells [9]. In RA patients, IL-12 p70 enhances IFN-γ dominant cytokine production by T cells infiltrating chronic arthritic joints, suggesting that IL-12 plays a crucial role in a shift toward rheumatoid T cells with Th1 cytokine profiles [10]. Patients with RA have significantly higher levels of IL-12 p70, a biologically active form of IL-12, in the sera and SF, and this increase was correlated with disease activity score [11]. It is well established that the main genetic determinant for RA susceptibility is correlated with specific alleles at the HLA-DRB1 gene [12]. However, genome-wide association studies (GWAS) have discovered more than 30 new RA susceptibility loci containing the IL-12 gene [13]. Growing evidence indicates that genetic factors such as SNPs play crucial roles in the pathogenesis of RA so genetic polymorphisms could be used as molecular biomarkers to predict disease development and progression [14]. Several studies have demonstrated an association between IL-12 gene polymorphisms and RA risk [15]. The frequency of a SNP in the gene encoding IL-12 is higher in RA patients compared with other autoimmune diseases [16], so IL-12B gene might be a functional candidate gene for some Th1-mediated autoimmune disorders, such as RA [3]. Therefore, our aim of this study is to improve knowledge about the genetic background of RA which would be a step on road to demonstrate the pathogenesis of the inflammatory process leading to the development of RA and to make its prevention feasible.
2. Subjects and Methods
2.1. Study Design
- This study was conducted between October 2016 and November 2017, it included thirty five patients with established RA who were recruited from outpatient clinics of Physical Medicine and Internal medicine departments of Al-Zahraa University Hospital. They were 4 males (11.4%), 31females (88.6%), their ages ranged from (23-58) years. Patients considered for this study were subjected to complete medical history taking, full clinical examination with special concern on musculoskeletal system, they were diagnosed as RA according to American College of Rheumatology (ACR) criteria (1987), they were fulfilling the 2010 ACR-EULAR classification criteria for RA [17]. Disease activity score (DAS) was determined for each patient according to DAS 28score [18]. Rheumatoid arthritis patients were compared with fifteen sex and age matched healthy individuals as a control group. Patient with other autoimmune diseases as Systemic lupus erythrematosis, Behcet disease, myasthenia gravis, bronchial athma, type 1 diabetes, chronic liver disease or those who receive biologic therapy were excluded from this study, all of them were free from cardiovascular diseases, infection, malignant disease, acute and chronic inflammatory diseases. An informed written consent was obtained from each patient and healthy subject after explaining the aim of the study.
2.2. Blood Sampling and Measurements
- Seven ml of venous blood was withdrawn from the antecubital vein of each individual and divided into three aliquots; 2 ml of whole blood was collected in sterile EDTA tube and stored at -20°C for further DNA extraction, another 2 ml of whole blood was collected in EDTA tube for ESR determination. The remaining part was collected in serum separator tube, centrifuged at 3500 rpm for 10 min then serum was divided into three portions; first one for turbidimetric measurements of CRP and RF using BioSystems kits Lot Number (19420, 16980, respectively). Second part was used for serum Anti-CCP measurement by quantitative sandwich enzyme immunoassay technique using INOVA Diagnostic, Inc (QUANTA Lite CCP3 IgG ELISA, with Lot Number: 028509). Third part of serum was stored at -20°C for further analysis of IL-12p70 which was measured by ELISA technique, kits were supplied by Bioassay Technology Laboratory Co., Ltd. with Catalog Number: E0099Hu and Lot Number: 170524-029. Measurement of serum IL- 12p70 was done on ELISA system (reader; A1851 Das, Italy, and washer; 16041412 Bio Tek, USA), according to manufacturer's instructions.
2.3. DNA Extraction and Genotyping
- Genomic DNA extraction from the stored blood was done by using Gene JET whole blood Genomic DNA purification Mini Kit #K0781 supplied by Thermo Scientific Co. Ltd, Lot Number 00459333. Extracted DNA was stored at -20°C prior to genotyping. The polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) method was used to determine the candidate SNPs in the IL-12B gene at position +1188A/C. The primer sequences were as follows: forward 50-CTG ATC CAG GAT GAA AAT TTG-30, reverse 50-CCC ATG GCA ACT TGA GAG CTG G-30. Amplification reaction was performed with 200 ng of genomic DNA in a 50-µl PCR mixture using 10 pmol of each primer, 0.25 mM each dNTP (Thermo Scientific), 1U Hot Star Taq Polymerase (Thermo Scientific #K1061, Lot No:00499215) and 19 PCR buffer (containing 1.5 µl M magnesium chloride, Thermo Scientific). The method for PCR included an initial denaturing at 95°C for 15 min, followed by 35 cycles at 94°C for 45 s, 54°C for 60 s and 72°C for 60 s with a final extension at 72°C for 10 min. The PCR product (226 bp) was digested with 1 µl of TaqI restriction enzyme (Thermo Scientific #ER0671, Lot: 00463436), separated in 2.5% agarose gel and visualized with ethidium bromide staining under ultraviolet light. Digestion resulted in a 226-bp full-length PCR product for allele A and 71- and 155-bp fragments for allele C.
2.4. Statistical Analysis
- Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges when their distribution found parametric, while qualitative data were presented as number and percentages. The comparison between two independent groups with qualitative data was done by using Chi-square test. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test. Pearson correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. Logistic regression analysis was used to asses predictors of RA with its odds ratio and 95% confidence interval. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at p < 0.05.
3. Results
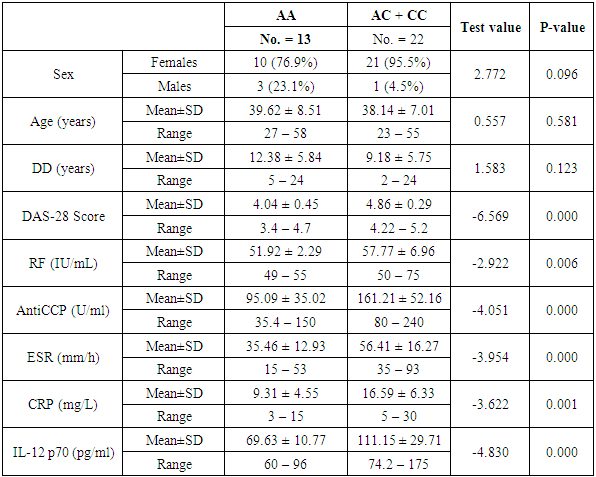
- The demographic and clinical characteristics of RA patients and control groups are shown in Table 1 and 2. Rheumatoid arthritis patients and control were matched for age and sex. The genotype distributions of IL-12B rs3212227 A/C in all subjects are illustrated in Table 3. Significant increase in frequencies of IL-12B gene rs3212227 AC and CC were found in RA patients as compared with control group (OR= 6.0, 95% CI 1.11-32.28, P = 0.026; OR= 8.307, 95% CI 0.911-75.73, P = 0.035) respectively, Table 3. The frequencies of AC+CC genotypes of the IL-12B gene rs3212227 were significantly higher between RA patients and control group (OR= 6.76, 95% CI 1.605-28.54, P = 0.005) Table 3. The frequency of (C) allele of IL-12B gene rs3212227 was significantly higher in RA patients as compared with control (OR=5.166, 95% CI 1.63-16.37, P = 0.003; Table 3). IL-12B (rs3212227) AC+CC genotypes were associated with higher DAS-28 score, RF, Anti-CCP, ESR, CRP and serum level of IL-12P70 (P = 0.000, P = 0.006, P = 0.000, P = 0.000, P = 0.001, P = 0.000) respectively Table 4. Serum level of IL-12p70 was significantly higher in RA patients as compared with control (P = 0.003) Table 2. Significant positive correlation was found between IL-12p70 serum level and DAS-28 score, RF, Anti–CCP, ESR, CRP (r = 0.853, p = 0.000; r = 0.724, p = 0.000; r = 0.674, p = 0.000; r = 0.691, p = 0.000; r = 0.640, p = 0.000) respectively Table 5, Fig. (1, 2, 3, 4, 5).
|
|
|
|
|
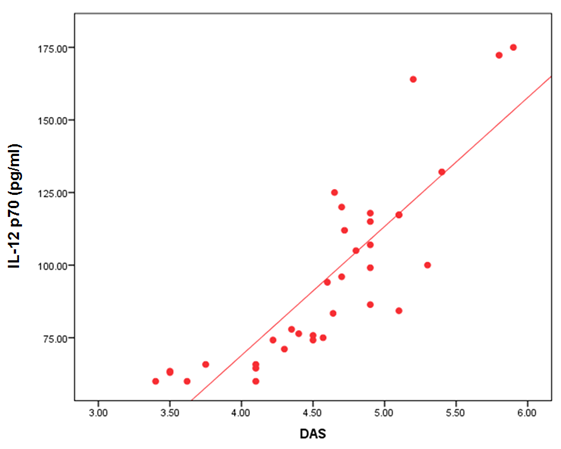 | Figure (1). Correlation between serum level of IL 12P70 and DAS-28 score |
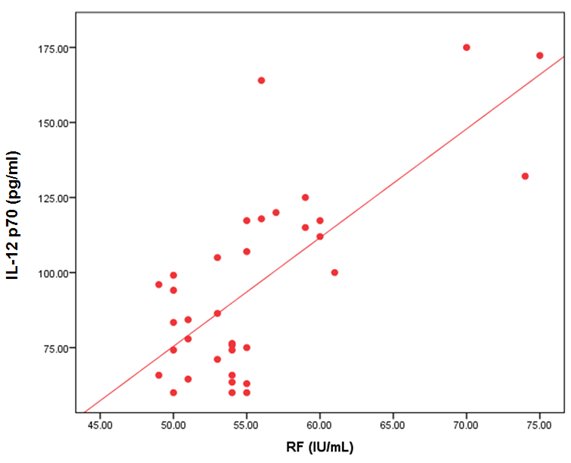 | Figure (2). Correlation between serum level of IL 12P70 and RF |
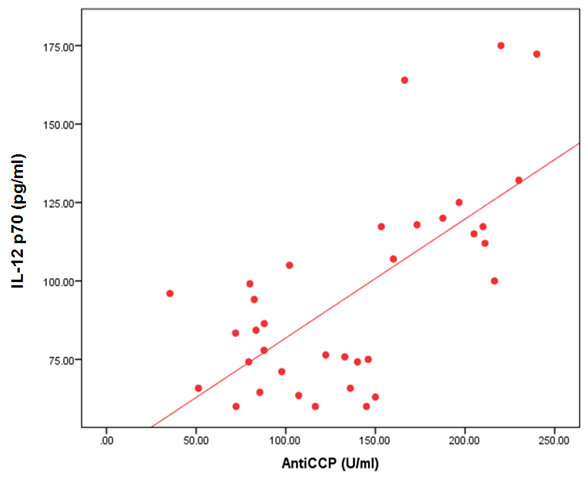 | Figure (3). Correlation between serum level of IL 12P70 and Anti-CCP |
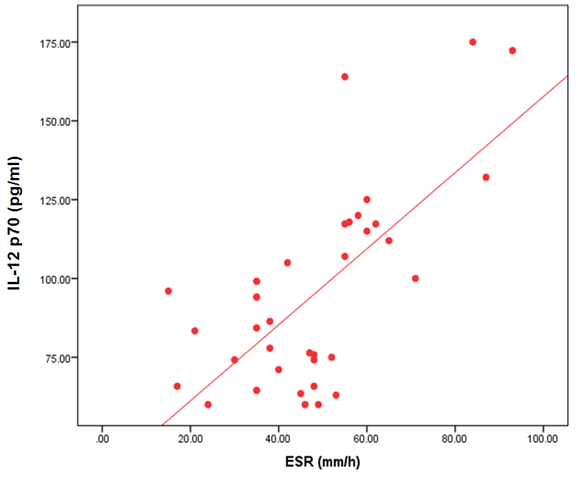 | Figure (4). Correlation between serum level of IL 12P70 and ESR |
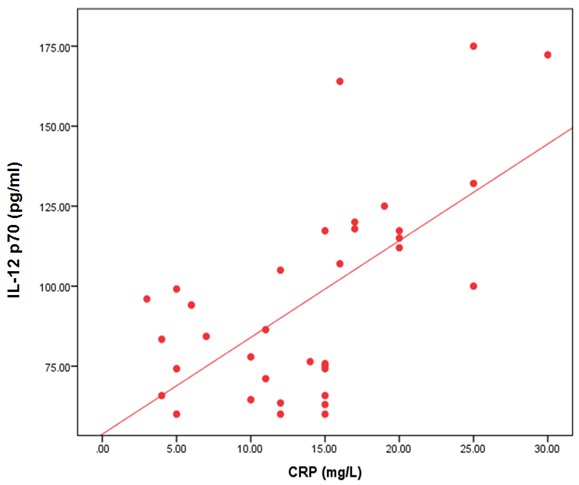 | Figure (5). Correlation between serum level of IL 12P70 and CRP |
4. Discussion
- Rheumatoid arthritis (RA) is a complex disorder, characterized by synovitis, progressive erosions, and cartilage destruction, it affects about 1% of the adult population world wide [19]. Although the etiology and pathogenic mechanism of RA are not fully known, some studies showed that the interaction between genetic characteristics and environmental triggers could influence susceptibility to RA [20]. Genetic factors such as SNPs play important roles in the pathogenesis of RA. The total heritability of RA was found to be approximately 66% [21]. Structural, quantitative or regulatory polymorphisms at the IL-12B locus may interfere with the course of T cell-mediated immune response and so, contribute to the susceptibility of some autoimmune diseases as RA [22]. It has been reported that IL-12B gene SNP rs3212227 was associated with the risk of many autoimmune and inflammatory diseases as type 1 diabetes [16], inflammatory bowel disease [13], asthma [23], Crohn's disease [24], psoriasis [25], multiple sclerosis [26].Genetic variants in the IL-12B gene were found to be associated with susceptibility of RA [15]. In the present study, the +1188A/C (rs3212227) polymorphism located in the 3′-untranslated region (3'-UTR) was chosen as it has biological significance in regulating protein synthesis [27]. In our study we found that the frequencies of IL-12B gene SNP rs3212227 AC/CC were significantly higher in RA patients as compared with control. At the allele level, we also found that the frequency of rs3212227 C allele was significantly higher in RA patients as compared with control, this result was in agreement with Paradowska-Gorycka et al. [22] who showed similar result. They demonstrated that polymorphism of +118A/C (rs3212227) which located in (3'-UTR) may have a biological importance, because this genetic variant may alter gene expression by changing the binding with IL-12 receptor and subsequently lead to defect in IL-12-induced signaling pathway. They concluded that IL-12B +118 A/C variant may be a susceptibility factor for RA.Our result also was matched with previous study done by Shen et al. [28] who found that IL-12B rs3212227AC and AC+CC genotypes were associated with risk of RA in older patients. Our data also was in accordance with Wang et al. [29] they demonstrated that IL-12B gene SNPs rs3212227A/C was associated with the development of RA and the frequency of (C) allele of IL-12B gene rs3212227 was associated with higher risk of RA. Individuals carrying the rs3212227 C/C haplotype were associated with a significantly increased risk of RA. On the other hand Varade et al. and Orozco et al. [30, 31] reported no association between IL-12B +118A/C (rs3212227) and RA. This discrepancies in genotype frequencies between studies may be explained by distinct ethnicities because, genetic polymorphisms often vary between ethnic groups, So, functional variations in IL-12 gene may contribute to the development of RA, and act as risk or protective factors of RA in other ethnic groups [28], and the limited sample size may be the causes of this discrepancies.We also found that IL-12B (rs3212227) AC+CC genotypes were associated with higher DAS-28 score, RF, Anti-CCP, CRP, ESR and serum level of IL-12P70, this result was in agreement of Wang et al. [29] who observed that Carriers of the CC genotype and the C allele of IL-12B gene rs3212227 were significantly more likely to be RF-positive and these patients were also more likely to develop severe RA. Also Shen et al. [28] reported that IL-12B (rs3212227) AC and AC+CC genotypes were found among RF positive patients, IL-12 plasma level of IL-12B rs3212227 C allele was significantly higher in RA patients than controls, and IL-12B rs3212227A/C allele might influence the inflammatory reaction of IL-12 in RA patients. Also, Seegers et al. [32] found that the presence of the C allele in IL-12B 3′UTR is associated with higher production of IL-12p70.Imbalance of cytokines originating from T lymphocytes has been associated with the pathogenesis of RA [4], IL-12p70 play a critical role in the differentiation of naive CD4+ T cells towards an IFN-γ-producing Th1-type cell in RA [8]. In the present study, we found that IL-12 p70 level was significantly higher in serum of patients with RA than those of healthy controls, which confirms previous reports done by Paradowska-Gorycka et al. [22] and Shen et al. [28] they found higher levels of IL-12 p70 in RA patients as compared with control and they demonstrated that, higher levels of IL-12 p70 may reflect the ongoing inflammatory process in RA patients.We found significant positive correlation between IL-12p70 serum level and DAS-28 score, Anti–CCP, RF, CRP and ESR, which was consistent with previous studies done by Kim et al. [4] they found that patients with detectable levels of IL-12p70 in sera had higher swollen joint scores, tender joint scores, and higher CRP level, they suggested that IL-12p70 production may be associated with an exacerbation of RA, and circulating IL-12p70 levels could be useful to assess disease activity. Our result was partially in agreement with Paradowska-Gorycka et al. [22] as they found that higher serum levels of IL-12 p70 was positively correlated with higher number of swollen, tender joints and CRP, however they found no association between IL-12p70 serum levels and ESR, RF, anti-CCP and frequency of IL-12B (rs3212227) A/C genotype. This discrepancy between the two studies may arise from racial variability, different study population sizes, and different patient's characteristic (e.g., age, disease duration and disease activity) and presumably, by the treatment they received.These observations suggest that RA patients with elevated IL-12 p70 levels may have marked disease activity and more activated macrophages, which produce more detectable cytokines, by immune cell interactions [33]. IL-12 also can induce the production of pro-inflammatory cytokines, including IL-1 and TNF-α, which contribute to the signs and symptoms of RA [4]. Also Ebrahimi et al. [34] proved that IL-12 serum concentration was more important and better predictive factor than TNF-alpha in RA course and in the active forms of the disease. So, IL-12 blockade could be useful for the treatment of RA [4].
5. Conclusions
- The identification of disease-associated interleukins and interleukin genes could provide precious insight into the genetic variations that is related to RA pathogenesis. SNP rs3212227 in IL-12B was associated significantly with an increased risk of RA and may be one of the candidate genes in the pathogenesis of RA. IL-12 p70 serum level can reflect RA disease activity, so IL-12 blockade could be useful for the treatment of RA. However, further well-designed studies with larger sample size and detailed information for both cases and controls are needed to confirm the conclusion.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML