-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2017; 7(5): 107-112
doi:10.5923/j.cmd.20170705.01

Clinical and Etiological Profile of Hypokalemia: A Prospective Study in a Tertiary Care Hospital
Suhail A. Malik1, Mosin S. Khan2, Syed Mudassar2, Parvaiz A. Koul1
1Department of Internal Medicine, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India
2Department of Clinical Biochemistry, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India
Correspondence to: Parvaiz A. Koul, Department of Internal Medicine, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Although limited data is available regarding the clinical and etiological spectrum of patients with hypokalemia in developing countries no study has systematically addressed the etiology of hypokalemia by adopting an algorithmic based approach. Keeping in view the nature and presence of hypokalemia in this region, a comprehensive study was undertaken to study the clinical and etiological profile of patients with Hypokalemia. One hundred forty-three (n=143) patients were included in the study with detailed examination and history. Biochemical analysis was done by Automatic Biochemistry and Immunoassay analyzer. Serum and urinary electrolytes along with blood gases were obtained in all patients using a blood gas analyzer. Urine osmolality was measured by freezing point method. On the basis of urinary K+ levels, extra renal loss (K+ < 15mml/l) was observed in 55.2% of patients compared to 44.8% having renal loss (K+ ≥ 15mml/l). Extra renal loss group (n=79) was further evaluated on the basis of Acid/Base status. Among the 79 patients 09% (07 of 79), 70% (55 of 79) and 21% (17 of 79) were having acidosis, normal pH and alkalosis respectively. Renal group was sub grouped on the basis of TTKG. 34 of 64 (53%) patients were having TTKG ≥ 4 compared to 30 out of 64 (47%) patients with TTKG < 4. In patients with TTKG ≥ 4 only 15 out of 34 (44%) patients were hypertensive compared to 56% (19 of 34) normotensive/hypotensive patients. A systematic algorithm based workup is recommended to decipher the potential cause and its elimination in the final management of hypokalemia.
Keywords: Hypokalemia, Algorithm, Renal loss, Urinary, Potassium
Cite this paper: Suhail A. Malik, Mosin S. Khan, Syed Mudassar, Parvaiz A. Koul, Clinical and Etiological Profile of Hypokalemia: A Prospective Study in a Tertiary Care Hospital, Clinical Medicine and Diagnostics, Vol. 7 No. 5, 2017, pp. 107-112. doi: 10.5923/j.cmd.20170705.01.
Article Outline
1. Introduction
- Normal serum potassium (K+) levels lie roughly between 3.5-5.5 mmol/L. The loss of just 1% (35mmol) of total body K+ would severely disturb the delicate balance between intra and extra cellular K+ concentration and lead to serious physiological consequences [1]. Hypokalemia is defined as serum K+ concentration <3.5 mmol/L, level between 3.5-3.0 mmol/L termed as mild hypokalemia, 2.9-2.5 mmol/L as moderate hypokalemia and a serum level of <2.5mmol/L as severe hypokalemia [2]. The frequency of hypokalemia in general population (in people who are not taking medication) is approximately less than 1%. Upto 21% of hospitalized patients have serum K+ lower than 3.5meq/L, with about 5% patients exhibiting K+ levels <3 mmol/L [3]. Hypokalemia can result from inadequate K+ intake (due to eating disorders, dental problems, poverty, poor parenteral nutrition) [4], intracellular shift (due to drugs, alkalosis, hypothermia, refeeding) [5], increased potassium secretion (due to mineralocorticoid excess, hyper-reninism, use of osmotic diuretics, increased gastrointestinal loss [6], hypomagnesemia or genetic disorders (congenital adrenal hyperplasia, Barter’s Syndrome, Gitelman syndrome, Liddle syndrome, Gullner syndrome, Hypokalemic periodic paralysis, SeSAME Syndrome, thyrotoxic periodic paralysis) [7]. Increased excretion is the most common cause, but several causes are often present [8].The symptoms of hypokalemia are non-specific and predominantly related to muscular or cardiac function [2]. With severe hypokalemia muscle cramps and pain (rhabdomyolysis) can occur [9]. Hypokalemia decreases potassium channel conductance, which lengthens repolarization time of a nerve cell. If severe enough, transmission of action potentials gets disrupted, resulting in generalized weakness or paralysis due to disrupting of the signaling pathway to the muscles. K+ depletion results in intracellular acidification and increase in net acid excretion or new HCO3 production. This is a consequence of enhanced proximal renal tubular HCO3 absorption, increased renal angiogenesis, and increased distal H+ secretion contributing to generation of metabolic alkalosis [10]. Severe potassium depletion may increase the risk of ventricular arrhythmias especially in patients with myocardial infarction or left ventricular hypertrophy [11].Hypokalemia with minimal renal K+ excretion suggest loss via routes other than renal. A 24hr urine K+ measurement establishes pathophysiologic mechanism of hypokalemia. Urine K⁺≤15mmol/L suggests a GI loss [6], poor intake [4] or a shift of the extracellular fluid K+ into intracellular space [5]. Conversely a high urine K+ (>15mmol/L) suggests a renal loss. Urinary Cl⁻ in patients having renal K⁺ loss can provide clue to diagnosis [6].While the data from the western and developed countries is sufficient, scant data are available regarding the clinical and etiological spectrum of patients with hypokalemia in developing countries even as it is a frequently encountered in hospitalized patients. No study has systematically addressed the etiology of hypokalemia by adopting an algorithmic based approach. Against this backdrop, our study is aimed to reveal the clinical and etiological profile of hypokalemia in patients attending the only tertiary care hospital of Kashmir valley.
2. Materials and Methods
2.1. Study Design & Period
- The present study was a hospital based prospective study in patients with hypokalemia, (defined as serum potassium below 3.5 mmol/L) conducted in the Department of Emergency Medicine in collaboration with the Department of Clinical Biochemistry, Sher-I-Kashmir Institute of Medical Sciences, Srinagar for a period of two (02) years from September 2014 to September 2016. The study has been approved by the Institute Ethical Clearance Committee (SKIMS).
2.2. Sample Size
- One hundred forty three (n=143) patients were recruited for the study. A detailed history with particular reference to symptoms and etiological factors of hypokalemia was obtained in all patients. All the patients underwent a detailed general physical and systemic examination.
2.3. Biochemical and Osmolality Estimation
- Biochemical analysis like serum urea, glucose, creatinine, bilirubin, AST, ALT, proteins, albumin, alkaline phosphatase was done on Beckman Coulter AU 680 automated analyzer (Beckman Coulter, Inc. © 2012, 250 S. Kraemer Blvd., Brea, CA 92821, USA). Serum levels of T3, T4, TSH, cortisol and aldosterone levels were assayed on Beckman Coulter Access 2 Immunoassay analyzer (Beckman Coulter, Inc. © 2013, 250 S. Kraemer Blvd., Brea, CA 92821, USA). Serum and urinary electrolytes along with blood gases was obtained in all patients. The analysis was done on GEM Premier 3000 Blood gas Analyzer using dry cartridge based chemistry (Instrumentation Laboratory, © 2002, 180 Hartwell Road, Bedford, MA 01730, U.S.A). Haemogram was obtained in all patients using Sysmex blood auto analyzer (Sysmex India Pvt Ltd., © 2008, 1002, Damji Shamji Business Galleria, LBS Marg, Kanjurmarg, Mumbai, India). USG abdomen and ECG was done in every patient and ECG changes were documented.In every patient Urine osmolality was measured by freezing point method [12] (reference) and serum osmolality was calculated. Trans tubular potassium gradient (TTKG) was calculated as per below given formula:
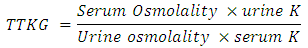
2.4. Diagnostic Algorithm
- According to predetermined algorithm on basis of urinary K⁺ patients were divided into two groups; renal loss (K+ >15mEq/L) and extra renal loss/intracellular shift (K+ ≤15mEq/L). In the extra renal loss group, on basis of serum pH, patients were further subdivided into acidosis, normal pH and alkalosis group. The acidosis group was further investigated for GI loss (vomiting, diarrhea). The normal pH group was further investigated for profuse sweating, drugs causing intracellular shift, hypomagnesemia, decreased intake and remote GI loses. The alkalosis group was investigated for remote diuretic use or GI loss.In the renal loss group the patients were further subdivided into two groups on basis of TTKG i.e. TTKG <4 and TTKG ≥4. Patients with TTKG <4 were investigated for use of osmotic diuretics, hypomagnesemia, GI loss. Patients with TTKG ≥4 were further subdivided on basis of blood pressure into hypertensive and normotensive/hypotensive groups. Among the hypertensive group serum aldosterone, 8am cortisol levels were measured to look for hypo or hyper aldosterone and hypo or hyper cortisol state and patients were further investigated for diuretic use or GI loss. The normotensive/hypotensive group was further subdivided into acidosis group and normal pH/alkalosis group. The acidosis group was further investigated for Diabetic Ketoacidosis (DKA), Renal tubular Acidosis (RTA), and amphotericin use. Urine pH was done in all patients of acidosis group. The normal pH/alkalosis group was further subdivided on basis of urinary chloride levels. Those with urinary Cl⁻ levels ≥20 mmol/L were further investigated for diuretic use and patients with urine Cl⁻<20 were investigated for GI loss of K⁺.Descriptive statistics was done for getting the mean, median etc. of the data set. The study was approved by the Institute Ethics Committee and informed consent was obtained from all the patients.
3. Results
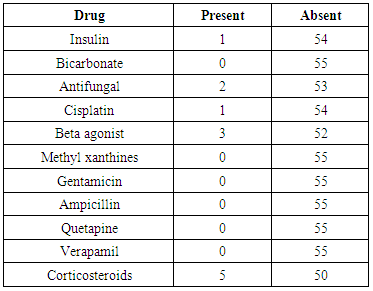
- The 143 enrolled patients consisted of 70 (49%) males, 73 (51%) females with a range of 13-80 years having median of 55 years. Hypokalemia was mild in 24 patients (17%), moderate in 82 (57%) patients and severe in 37 (26%) patients. Among mild hypokalemia only 01 patient had fatigue and 02 patients had muscle weakness. In moderate hypokalemia 02 patients had fatigue, 08 patients had muscle weakness, 03 patients had exercise intolerance, 02 patients had palpitations, 03 had constipation. In severe hypokalemia 04 patients had fatigue, 08 patients had muscle weakness and only 01 patient had exercise intolerance (Figure 1). In mild hypokalemia 01 patient had grade I and 01 patient had grade III muscle power. In moderate hypokalemia 03 patients had grade I, 03 had grade II and 03 had grade III muscle power. In severe hypokalemia 04 patients had grade I, 03 patients grade II and 01 had grade III power. No specific ECG change was found in 63% of patients, U waves in 23%, T wave flattening in 8%, wide QRS in 4%, T wave inversion in 2% of patients. In mild hypokalemia 2 patients had U waves. In moderate hypokalemia 14 patients had U waves, 06 patients had T wave flattening, 01 patient had T wave inversion and 03 patients had wide QRS complex. In severe hypokalemia 18 patients had U waves, 06 patients had T wave flattening, 02 had T wave inversion and 04 had wide QRS complex (Figure 2).
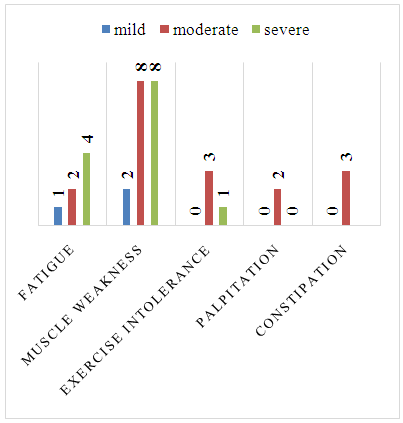 | Figure 1. Symptoms attributable to hypokalemia |
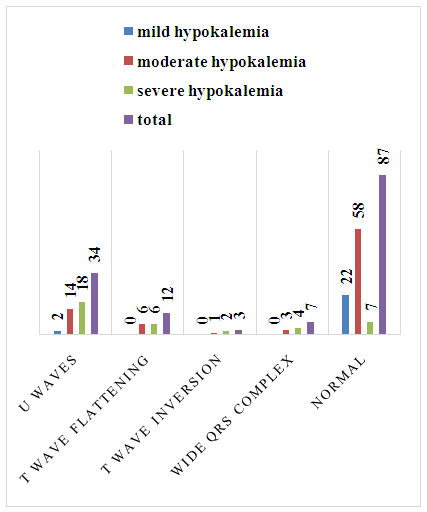 | Figure 2. ECG changes in hypokalemia |
|
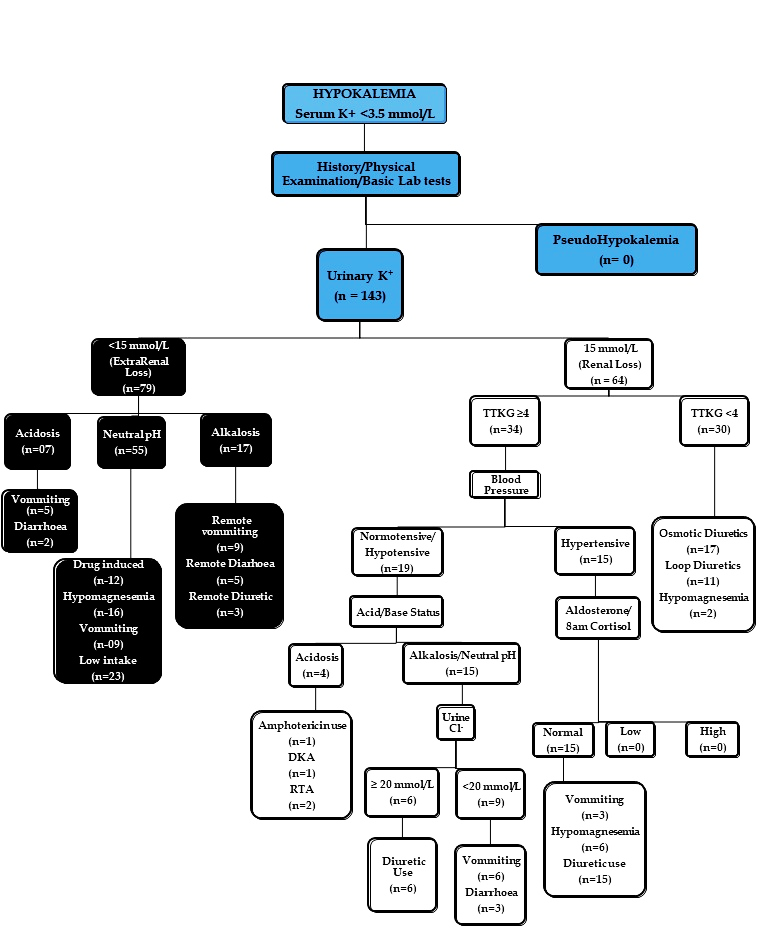 | Figure 3. Diagnostic Algorithm of Hypokalemia (DKA; Diabetic Ketoacidosis, RTA; Renal tubular Acidosis, K+; Potassium, Cl+; Chloride, TTKG; Trans tubular potassium gradient.) |
4. Discussion
- Our data including 143 patients describes the clinical features and etiology of hypokalemia, as seen in a tertiary care hospital. This happens to be the first systematic study on etiological and clinical features of hypokalemia in India. No previous study is available that has addressed the clinical and etiological profile of hypokalemia across all severities of the electrolyte disorder. Most other studies on hypokalemia have mostly focused on only one aspect of hypokalemia [13].In our study, on taking into account the history, physical examination and Basic lab investigations none of the patient was found to have Pseudo hypokalemia and hence excluded from the study. Symptoms because of hypokalemia are not often seen with serum potassium levels above 2.5 mmol/L [14]. In our study, only one fourth (24%) of patients with serum potassium levels of 3.5 mmol/L or lower presented with symptoms attributable to hypokalemia. In mild hypokalemia patients 12% were symptomatic, in moderate hypokalemia patients 22% were symptomatic and in severe hypokalemia 35% were symptomatic. Marti et al. found 50% patients showing symptoms attributable to severe hypokalemia with muscle weakness being the most common symptom present in 36% of patients [13]. Muscle weakness in 18 patients (13%) and fatigue in 7 patients (5%) were the leading symptoms of hypokalemia in our patients too. Hypokalemia decreases potassium channel conductance, which lengthens repolarization time of a nerve cell. If severe enough, transmission of action potentials gets disrupted, and resulting in generalized weakness or paralysis because signaling pathway to the muscles are disrupted [10].ECG changes because of hypokalemia were seen in 39% (56 of 143) of our patients. The presence of U waves, T wave flattening and wide QRS complex were the most common ECG changes seen in over 35% of patients. In mild hypokalemia patients 8% showed ECG changes, in moderate hypokalemia patients 29% showed ECG changes and in severe hypokalemia patients 81% showed ECG changes. Marti et al. found 69% patients showing ECG changes attributable to severe hypokalemia with a U wave being most commonly present in 24% of patients, followed by ST segment depression in 21% of patients. The slightly higher incidence of ECG changes in their study could be attributed to the fact that they had included patients only with severe hypokalemia. Joel et al. found the earliest electrocardiogram (ECG) change associated with hypokalemia is a decrease in the T-wave amplitude [15]. As potassium levels decline further, ST-segment depression and T-wave inversions are seen. A low serum potassium concentration leads to a more negative resting membrane potential; decrease in membrane excitability; increase in the action potential duration and a delay in repolarization [16]. Typical ECG manifestations of hypokalemia include flattened T waves, prominent U waves, ST segment depression, and a fusion of T and U waves. Also, an increase in QRS duration, atrioventricular block, or cardiac arrest may occur [17, 18]. Hypokalemia is also a known cause of prolonged QT syndromes and associated ventricular tachyarrhythmia’s.Diuretic medication, vomiting, low oral intake and hypomagnesemia were the main causes for hypokalemia in our patients. Diuretic intake was causative factor of hypokalemia in 38% of our patients. The patients in our study were clearly sick on account of their admission through Emergency Department and the etiological profile may not be representative of the etiological factors operative in hypokalemia patients. Janko et al. also found previously that main causes of hypokalemia were diuretics and gastrointestinal potassium loss [19].Low oral intake was causative factor of hypokalemia in 23% of our patients. Fasting or low intake may be a significant contributor to the development of hypokalemia in patients under diuretic medication, with a reduced capacity of renal counteraction [20].Hypomagnesemia was the causative factor of hypokalemia in 17% of our patients. Magnesium deficiency impairs Na-K-ATPase, which would decrease cellular uptake of K⁺. At the physiological intracellular Mg⁺⁺ concentration ROMK (renal outer medullary potassium) channel in cortical collecting duct (CCD) conduct more K⁺ ions inward than outward (inward rectifying). This is because intracellular Mg⁺⁺ binds ROMK and blocks K⁺ efflux. Influx of K⁺ ions displaces intracellular Mg⁺⁺, allowing maximal K⁺ entry. This unique inward-rectifying property of ROMK places K⁺ secretion in the distal nephron under the regulation by intracellular Mg⁺⁺. In hypomagnesemia ROMK acts in reverse direction [21]. Recent evidence from patients with cardiac diseases, renal diseases showed that hypokalemia was an independent risk factor for adverse outcome in form of increased morbidity and mortality. Joseph et al. found hypokalemia was associated with a 2.5-fold increase in relative risk for cardiac arrest [22].
5. Conclusions
- Despite the small sample size ours is the most comprehensive study of clinical features and etiological profile of hypokalemia in the country, where a systematic enquiry into the etiology of hypokalemia was conducted based on well described algorithmic plan of investigations. Clinical features may not be predictive of a particular cause and severity of hypokalemia and as such a systematic algorithm based workup is recommended to decipher the potential cause and its elimination in the final management.
ACKNOWLEDGEMENTS
- We greatly acknowledge the technical staff of the department of internal medicine and clinical Biochemistry who helped a great deal in collection and processing of samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML