-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2017; 7(3): 75-79
doi:10.5923/j.cmd.20170703.03

Evaluation of Dried Blood Spots as an Alternative Sample Type in HIV-1 RNA Quantification from Patients Receiving Anti-Retroviral Treatment in Kenya
Edward M. Onkendi1, 2, Dorcus A. Abuya1, Ruth M. Mumo1, Leonard King’wara1, Geoffrey K. Kangogo1, Stephen K. Bera1, Nancy N. Bowen1
1Division of National Public Health Reference Laboratories (NPHLS), National Virology Unit & National HIV Reference Laboratory (NHRL), Nairobi, Kenya
2Department of Microbiology, University of Pretoria, Pretoria, South Africa
Correspondence to: Edward M. Onkendi, Division of National Public Health Reference Laboratories (NPHLS), National Virology Unit & National HIV Reference Laboratory (NHRL), Nairobi, Kenya.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Routine HIV-1 viral load monitoring for patients under anti-retroviral therapy is still a challenge in developing countries due to limited access to healthcare facilities and testing services. One of the key challenges hampering access to testing services is the intricate logistics associated with plasma samples required for viral load monitoring. Therefore, there is need to overcome these challenges through new technologies which are affordable and easy to implement. In this validation, EDTA blood samples from 180 HIV-1 patients receiving anti-retroviral therapy were used to prepare dry blood spots and plasma samples for HIV-1 RNA measurements using Roche Cobas Taqman assay. In general, HIV-1 RNA measurements in DBS were lower than those obtained in plasma samples. Detection rates in DBS were 100% in plasma samples with ≥ 3.0 log10 copies/mL. However, plasma samples with ≤ 2.3 log10 copies/mL were not detected in DBS. There was a high correlation (Pearson’s correlation, r = 0.917, P < 0.05) in HIV-1 RNA measurement between dry blood spots and plasma samples. When 12 DBS samples were tested for viral copy numbers after three weeks of storage at room temperature (24°C), there was a high correlation (Pearson’s correlation, r = 0.906, P < 0.05) in HIV-1 RNA measurement between dry blood spots and plasma samples. In addition, there was good concordance between individual plasma and dry blood spot results based on Bland and Altman analysis. Overall, results from this validation suggest that DBS can be used as an alternative sample type for HIV-1 RNA measurements in ART care patients.
Keywords: Dried blood spots, Plasma, Resource-limited, HIV-1 RNA, Regimen failure, Kenya
Cite this paper: Edward M. Onkendi, Dorcus A. Abuya, Ruth M. Mumo, Leonard King’wara, Geoffrey K. Kangogo, Stephen K. Bera, Nancy N. Bowen, Evaluation of Dried Blood Spots as an Alternative Sample Type in HIV-1 RNA Quantification from Patients Receiving Anti-Retroviral Treatment in Kenya, Clinical Medicine and Diagnostics, Vol. 7 No. 3, 2017, pp. 75-79. doi: 10.5923/j.cmd.20170703.03.
Article Outline
1. Introduction
- Roll-out, access and uptake of anti-retroviral therapy (ART) for patients infected with human immunodeficiency virus (HIV-1) have improved significantly in the past decade in Sub-Saharan Africa [1]. The WHO progress report of April 2007 highlighted that 28% of the estimated 7.1 million people living with HIV/AIDS in low and middle income countries were in need of ART by the end of 2006 [1]. Although the number of people accessing ART care in Kenya has increased remarkably over years, a significant segment (approximately 900,000) of adults (15 years and above) living with HIV-1 are in need of these services (December, 2016 HIV estimates, Kenya). Furthermore, there is anecdotal evidence showing that treatment based only on immunological diagnostic results rather regimen failure due to drug resistance mutations are not effective [2]. Therefore, there is need to adopt simple, effective and accessible methods of diagnostics to evaluate regimen failure in patients receiving ART in the resource-limited settings of Kenya. Over years, plasma has been used as the most appropriate sample type (gold standard) for monitoring regimen failure in HIV-1 patients attending ART care. However, collection, storage and cold chain transportation of plasma samples for HIV-1 virologic monitoring poses significant challenges to resource-limited peripheral laboratories in Sub-Saharan Africa [3]. To circumvent the logistical challenges associated with plasma, dried blood spot (DBS) has been proposed as the suitable alternative sample type for HIV-1 viral load determination [4-7]. Dry blood spots can be collected easily, stored at room temperature and shipped to testing laboratories at a later date [8-10]. Furthermore, the detection limit, accuracy, challenges and successes of using DBS for HIV-1 RNA measurement has been discussed extensively in previous studies [11-15].Dried blood spots have been used successfully to offer HIV-1 testing services in different sub-Saharan countries [16-19]. Therefore, if the DBS technique is well adopted, it can expand accessibility of HIV-1 testing services in resource-limited settings. In this validation, we sought to evaluate the efficiency and accuracy of using dry blood spots for virologic monitoring in patients receiving ART care from two healthcare facilities in Kenya. We further evaluated the stability of stored DBS samples in viral load measurements using the Roche TaqMan Assay.
2. Materials and Methods
- Study site and populationBetween August and December 2016, DBS and plasma samples were collected from whole blood in EDTA purple-top vacutainers drawn from 180 HIV-1 patients attending routine ART care at Mbagathi District Hospital and Kenyatta National Hospital in Nairobi. The subjects included 61% women and 39% men between the ages of 4-73 years. Enrolled patients were either ART experienced or naïve and the experienced ones were still either on their first or second line treatment regimen. All HIV-1 infected patients were from the surrounding areas of Nairobi County where our reference laboratory is located. All patients/guardians were requested to sign written consent forms before samples were collected and the entire procedure was done as per the guidelines of Good Clinical Laboratory Practices (GCLP) required for method validation. Moreover, since this is part of operational research at the National HIV Reference Laboratory, ethical clearance was provided by African Medical Research Institute (AMREF).Specimen collection and preparationA four millilitre blood sample was collected from each patient by venous puncture into EDTA containing tubes. To prepare dry blood spots, all blood samples were mixed gently after collection and 70 µL spotted on each of the five circles of the 903 Whatmann filter papers (Schleicher & Schuell, BioScienceGmbH, Barcelona, Spain). Previous studies showed that 70 µL of whole blood spotted on DBS card is optimal for HIV-RNA quantification (unpublished data). The cards were left to dry for at least 4 h at 24°C, packed in zip-lock bags containing a desiccant and shipped to the National HIV Reference Laboratory (NHRL). Plasma was separated from the collected whole blood by centrifuging the remaining blood in EDTA tubes at 2500 rpm for 10 min. From each sample, approximately 1500 µL plasma was collected and sent frozen to NHRL. All plasma samples were stored at -80°C until processing.Plasma processing for HIV-1 RNA quantificationAutomated extraction of RNA and quantification of Copies of HIV-1 RNA from plasma samples were done using the Roche Cobas Taq Man platform (Roche, Basel, Switzerland) according to the manufacturer’s instructions. The lowest detection limit was set at 40 copies per mL. Plasma sample results were used to select DBS samples for HIV-1 RNA quantification. Only DBS samples whose plasma had ≥1000 copies/mL were selected for analysis. DBS processing for HIV-1 RNA quantificationTo quantify HIV-1 RNA from DBS samples, one pre-punched 12 mm spot (containing 70 µL of whole blood) from the DBS card was obtained using sterile forceps and placed in the sample tube. Next, 1000 µL of phosphate buffered saline, pH 7.2 (free from MgCl2 and CaCl2) was added to each sample and left to stand at room temperature for 1 h. After incubation, the tubes containing the DBS samples were finger-tapped gently to ensure complete elution before HIV-1 RNA was evaluated using the manufacturer’s instructions on the Cobas Taq Man platform (Roche, Basel, Switzerland). In this assay, positive and negative controls were the ones supplied by the manufacturer for HIV-1 RNA quantification in plasma.Reproducibility and stability of HIV-1 RNA copy numbers in DBS samplesAll DBS samples which were stored at room temperature for three weeks were used to select a cohort to test the reproducibility and stability of HIV-1 RNA copy numbers. Twelve DBS samples were randomly selected, eluted according to the established protocol and HIV-1 RNA copy numbers measured according to the manufacturer’s instructions on the Cobas Taq Man platform (Roche, Basel, Switzerland). The results were compared to the corresponding plasma values obtained initially.Statistical analysisAll statistical analyses were performed using the statistical software JMP v 5 (SA Institute Inc., Cary, NC, USA). All HIV-1 RNA results per mL of blood for plasma and DBS were log10 transformed and used for calculations in this study. To compare the results for each sample type, regression analysis was performed and the Pearson’s correlation was evaluated based on the R value. Furthermore, concordance between plasma and DBS results was investigated using Bland and Altman analysis.
3. Results
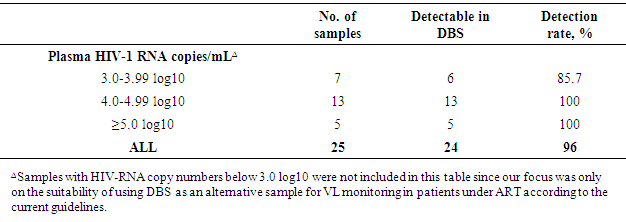
- Study population Of the sampled ART patients, six were on second line treatment while the rest were on first line. Only one patient on second line since 2008 had viral copy numbers above 1000. In addition, one pregnant patient on second line since 2010 had viral copy numbers above 1000. Overall, the sampled population was diverse in age, dates of ART care enrolment and suppression levels of the HIV-1 virus. All collected samples were processed for DBS and plasma at the respective facilities before being analysed at NRHL on the Roche Cobas TaqMan platform.Analysed plasma samples were clustered into three broad groups based on the log10 values obtained from them. The broad groups included samples with a range of 3.0-3.99 log10 (n=7), 4.0-4.99 log10 (n = 13) and ≥5.0 log10 (n = 5). The three broad groups were thereafter used to determine the detection rate in DBS samples (Table 1).
|
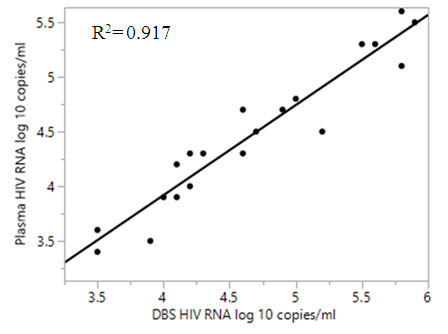 | Figure 1. Regression analysis of 23 plasma and DBS samples after collection |
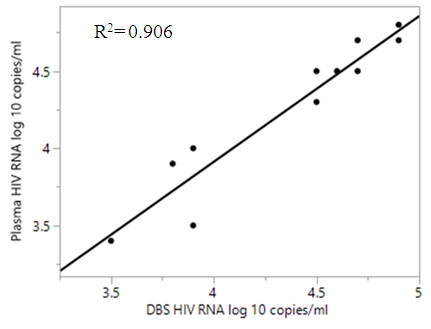 | Figure 2. Regression analysis of 12 select plasma and DBS samples three weeks after collection |
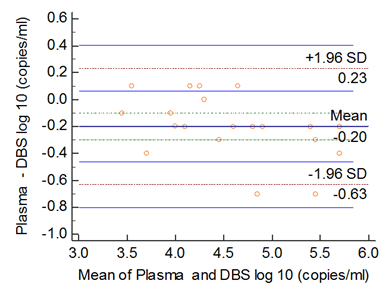 | Figure 3. Bland and Altman analysis of 23 DBS and plasma samples |
4. Discussion
- The success of field and laboratory evaluation of dry blood spot as an alternative and reliable sample type for monitoring virological failure in ART initiated patients will be a great step towards achieving the 90-90-90 UNAIDS targets in resource-constrained settings. Several studies have evaluated the possibility of using dry blood spots for HIV-1 RNA quantification in ART initiated patients using but not limited to Roche Cobas TaqMan platform [6, 9, 12, 13, 17, 20-22]. Both studies registered a higher level of correlation between dry blood spots and plasma when used in HIV-1 RNA quantification. For example, when using dry blood spots and plasma for viral load monitoring on Roche Cobas TaqMan platform, Andreotti et al. (2010) reported a high correlation (R2 = 0.96, p = 0.001) and a 96.4% detection rate for DBS samples with ≥ 3.0 log10 copies/mL in their corresponding plasma.In this assay, we adopted the free virus elution (FVE) protocol developed by Roche Inc. to guarantee quality results. In this protocol, sample preparation was subjected to a strict and well adhered to procedure, assayed and preliminary DBS results obtained entered into an FVE calculator with a correction factor. The final DBS results for all processed samples were then compared to their corresponding plasma values. With the strict adherence to the FVE protocol, we recorded a very good agreement between DBS and plasma HIV-1 RNA values for copy numbers/mL. The detection rate in DBS samples was quite high (96%) for samples which are used to determine the success or failure of ART care. In addition, detection rate peaked to 100% as the viral copy numbers in DBS samples increased past 3.99 log10. When stability of the DBS samples was evaluated after storage for two weeks it was observed that the time lapse did not affect the accuracy of the HIV-RNA viral copy numbers. All DBS samples were stable under the described storage conditions and this is a good observation since peripheral labs which are resource constrained can opt for DBS instead of plasma samples. Preparation and transportation of DBS samples does not require special equipment and cold-chain shipment thus reducing the logistical challenges often posed by plasma samples. This investigation further strengthens the possibility of processing DBS samples stored for two weeks at 24°C or -20°C (for long term) without compromising the quality of results. In this validation, we did not yield any false positive in DBS samples. However, one sample with 3.6 log10 copies/mL in plasma was not detected in the corresponding DBS sample. We further observed that one sample with 3.2 log10 in plasma was detected as < 400 copies/mL in the corresponding DBS sample. Despite these two cases, in general, the performance of DBS samples for viral load testing was very good as evidenced by detection rate and stability. Of the 23 DBS processed, 14 recorded slightly more HIV-1 RNA copies/mL compared to their corresponding plasma samples albeit the mean difference in this category of samples was 0.2 log10. We hypothesize that the slightly more copies of HIV-1 RNA in DBS may have been influenced by the proviral DNA in the whole blood used to prepare the samples.
5. Conclusions
- Most of the HIV-1 affected countries in the Sub-Sahara are resource-limited. Furthermore, ART care has not been accessed by all people living with HIV-1 and, even for those who have been initiated, a significant proportion is failing virologically [1]. Therefore there is need to design simple, accurate and accessible methods of monitoring those who are failing ART care. Currently, virologic failure in ART initiated patients is based on HIV-1 RNA measurements using plasma samples (as the gold standard) on different technologies [12, 19, 23].This validation demonstrates the possibility of using DBS as an alternative sample type for virologic failure monitoring in patients under ART care. The correlation between DBS and plasma samples was high (Pearson’s correlation, r = 0.917, P < 0.05) in HIV-1 RNA measurement. This was further supported with a high correlation (Pearson’s correlation, r = 0.906, P < 0.05) when 12 DBS samples were randomly tested after three weeks of storage at room temperature (24°C). The high correlation between DBS stored for three weeks and plasma affirms that stability can be guaranteed in this sample type if the right procedures are adhered to.
ACKNOWLEDGMENTS
- We thank the Ministry of Health, Kenya, PEPFAR, Global Fund and other supporting partners which we cannot enlist all here for funding this validation. We also thank the technical staff from Mbagathi District Hospital and Kenyatta National Hospital for their support during this validation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML