-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2017; 7(3): 67-74
doi:10.5923/j.cmd.20170703.02

Relationship of Serum BAFF Levels and BAFF Receptor (BAFF-R) Expression on B Lymphocytes in Early and Late Rheumatoid Arthritis Egyptian Patients
Amany M. Elsaeed1, Entsar R. Mokhtar1, Eman M. Abdelsalam2, Mona El-fedawy EL-Saied2, Hala M. Nageb2
1Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Amany M. Elsaeed, Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Rheumatoid arthritis (RA) is a common autoimmune disease that is marked by a systemic inflammatory reaction and joint erosions. B cells play an important role in the perpetuation of RA, particularly as autoantibody producing cells. B cell activating factor (BAFF) is a key survival factor during B cell maturation and is essential for the development of B cell tolerance. Breakdown of the regulation of BAFF expression results in excessive BAFF production, that impairs B cell tolerance and leads to autoimmune phenomena. Elevated levels of BAFF have been detected in the serum and synovial fluid of RA patients, thus BAFF has become a very attractive target for the treatment of RA with an altered B cell function. Objective: To assess levels of serum BAFF (sBAFF) and expression of BAFF receptor (BAFF-R) in RA patients either early or late. Methods: The present study included 30 patients with RA who were divided into two groups: 15 patients with early RA (ERA) and 15 patients with late RA (LRA). They were compared with 15 sex and age matched healthy individuals as a control group. Serum BAFF levels of all groups were measured by ELISA, while BAFF-R expression on peripheral blood B lymphocytes were examined by flowcytometric analysis. Results: There was a highly significant increase in sBAFF levels in patients with ERA when compared with LRA (P=0.000) and control (P=0.000), while BAFF-R expression was significantly higher in LRA when compared with ERA patients (P=0.000) and control (P=0.000), but no significant difference when compared ERA patients with control (P=0.643). Conclusion: The dysregulation of BAFF/BAFF-R system may contribute to the induction and development of RA. Therefore, BAFF/BAFF-R targeting therapy is a promising approach to treat B cell related autoimmune disease e.g. rheumatoid arthritis.
Keywords: Rheumatoid arthritis, B cells, Serum BAFF, BAFF Receptor
Cite this paper: Amany M. Elsaeed, Entsar R. Mokhtar, Eman M. Abdelsalam, Mona El-fedawy EL-Saied, Hala M. Nageb, Relationship of Serum BAFF Levels and BAFF Receptor (BAFF-R) Expression on B Lymphocytes in Early and Late Rheumatoid Arthritis Egyptian Patients, Clinical Medicine and Diagnostics, Vol. 7 No. 3, 2017, pp. 67-74. doi: 10.5923/j.cmd.20170703.02.
Article Outline
1. Introduction
- Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation predominantly affecting synovial joints and a high risk of inflammation induced irreversible joint damage [1]. RA patients often present with symmetric polyarticular arthritis that primarily affects the small diarthrodial joints of the hands and feet [2].Although the etiology of RA is incompletely understood, B cells contribute to the disease process by playing a combination of roles from T-cell activation being as Ag presenting cells (that leads to production of proinflammatory chemokines and cytokines such as IFN-γ IL-6 or IL-10), being precursors of plasmablasts and inducing plasma cells to secrete autoantibodies [3]. It is widely accepted that B cells play an essential role in the pathogenesis of RA. B cell involvement is well documented in the presence of detectable autoantibodies in the majority of patients, in particular rheumatoid factor and antibodies against citrullinated proteins (ACPA) which can be detected many years before development of clinical disease indicating that autoreactive B cell clones are involved in disease induction [4].B cell activation and maturation are under the control of soluble and membrane bound B cell activating factors that belong to the tumor necrosis factor (TNF) superfamily. An innate cytokine BAFF (B cell activating factor) also called BLYS (B lymphocyte stimulator) has emerged as crucial factor that modulates B cell survival, activation, maturation, tolerance and homeostasis [5]. BAFF is predominantly produced and released by myeloid cells, notably monocytes, neutrophils, macrophages, dendritic cells, T and B cells [6].BAFF is produced as transmembrane protein, which cleaved at a furin protease site and then released in a soluble form. BAFF also remains active as a membrane bound form, although the soluble form is required for B cell homeostasis [7].BAFF is a ligand for three TNF receptor superfamily members: B cell maturation antigen (BCMA), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI) and BAFF-R. Among them BAFF-R plays the central role in the BAFF system. BAFF/BAFF receptors appear to span nearly all stages of B-lineage differentiation ranging from the development, selection and homeostasis of naïve primary B cells to the maintenance of long-lived bone marrow plasma cells. BAFF-R is absent on B cell precursors in the bone marrow and their expression increases as B-lineage cells develop in the periphery and is critical for immature B cell survival [8].There is much interest in the role of BAFF and its receptor (BAFF-R) in the pathogenesis of autoimmune disease in terms of auto-reactive B cell survival and function [9]. Increased expression of BAFF might explain pathogenic B cell activation in RA. Interestingly, autoreative B cells depend more on BAFF for survival than do alloreactive B cells [10].In the light of these data, the aim of the present study was to investigate the levels of sBAFF and the expression of its receptor (BAFF-R) on B cells of ERA and LRA patients as well as their roles in these patients.
2. Subjects and Methods
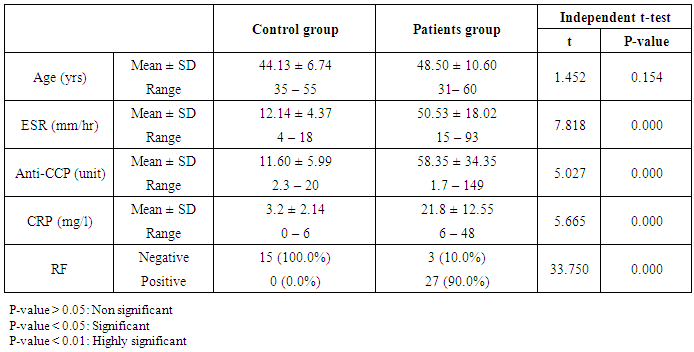
- This study included thirty patients with RA recruited from inpatient and outpatient clinics of the Internal Medicine Department-Rheumatology division of Al-Zahraa University Hospital during the period from April 2016 to December 2016. They were fulfilling the new 2010 EULAR/ACR criteria for RA [11]. Fifteen age and sex matched apparently healthy subjects were selected as a control group. An informed consent was taken from each patient and control after explaining the purpose of the study, which is approved by the local ethics committee. Patients with other autoimmune disorders affecting serum BAFF or BAFF-R as SLE, Behcet disease, myasthenia gravis, bronchial asthma, chronic liver diseases and type 1 diabetes or those receiving any biologic therapy were excluded and all patients were free of infection, malignant diseases, cardiovascular complaints or other inflammatory diseases.Patients were classified according to disease duration into ERA patients who had RA symptoms for more than six weeks and less than one year and LRA patients with disease duration for more than one year [4]. All patients and control group were females, the age of patients ranged from (31-60) years.Seven ml of venous blood were withdrawn from each subject and divided into three portions: 2 ml of whole blood was collected in evacuated tube containing EDTA as anticoagulant which was sent immediately for the lab for flowcytometric analysis of (BAFF-R) and 2 ml collected on EDTA for ESR measurement. The remaining part was centrifuged and serum was divided into two portions, the first one for CRP (semiquantitative) and RF assessment which was done by latex agglutination test: Biomed Diagnostics lot no. 110515, the second part was stored at -20˚C until be used for Anti-CCP and serum BAFF measurements. Anti-CCP was assessed by enzyme quantitative immunoassay using INOVA Diagnostic, Inc (QUANTA Lite CCP3 IgG ELISA 704535) on ELISA system (Reader A3 1851 & Washer 909) from Das (Italy).
2.1. Serum BAFF Measurement
- It was done using enzyme quantitative immunoassay using Quantikine® ELISA (Human BAFF/BLyS/TNFSF13B Immunoassay-Catalog Number (DBLYSOB, SBLYSOB and PDBLYSOB) according to the manufacturer's instructions. Samples were analysed on ELISA system (Reader A3 1851 & Washer 909) from das (Italy).
2.2. Flowcytometric Analysis of BAFF-R
- It was done on Becton Dickinson Facs caliber flowcytometry with a standard 4-color filter configuration using R&D system Kits.Reagents used were monocolonal antibodies that include human BAFF-R (TNFRSF13C) PE-conjugated Antibody (lot Number: ADQM01130061) and human epitope CD 19 FITC-conjugated Antibody (lot Number: AAML0214091). Control used was FITC & PE istoype control (lot Number: 28206). Contaminating serum components were removed from whole blood by washing the cells three times in an isotonic phosphate buffer (PBS 0.5%) by centrifugation at 500xg for 5 minutes.Two tubes, each contain 50 µL of packed cells were set for each subject. One for the tested monoclonal Abs and the other tube for isotype control to detect nonspecific binding for auto- fluorescence for cells used. Ten micron of human anti BAFF-R PE-conjugated, and Human CD19 FITC-conjugated antibody reagents were added and incubated for 20 minutes at room temperature in a dark place. One ml of lysing reagent was added for 10 minutes in dark place, followed by centrifugation at 1300 rpm for 5 minutes at room temperature, then supernatent was discarded and the pellet was left. The pellets were washed twice with 3 ml of PBS buffer, and the cells were resuspended in 200 µl of cell sheath fluid for final flow cytometerical analysis. Data acquistation and analysis were performed on EPICSXL flowcytometry using system software with a standard 4-color filter configuration. Lymphocytes were gated via their forward and side scatter properties, then B cells were identified based on their expression of CD19. The identified B cells were assessed for BAFF-R expression.
3. Statistical Analysis
- Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 20. The qualitative data were presented as number and percentages while quantitative data were presented as mean, standard deviation and ranges when their distribution found parametric and were presented as median with interquartile ranges (IQR) when their distribution found non-parametric. The comparison between two groups with qualitative data were done by using Chi-square test and/or Fisher exact test was used instead of Chi-square test when the expected count in any cell was found less than 5. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test while data with non parametric distribution compared between two groups using Mann-Whitney test. The comparison between more than two independent groups with quantitative data and parametric distribution was done by using One Way Analysis of Variance (ANOVA). Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following: P > 0.05: Non significant; P < 0.05: Significant; P < 0.01: Highly significant.
4. Results
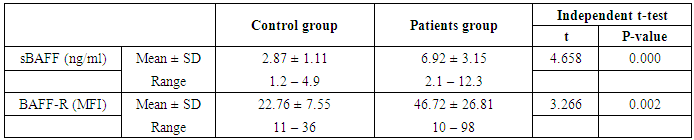
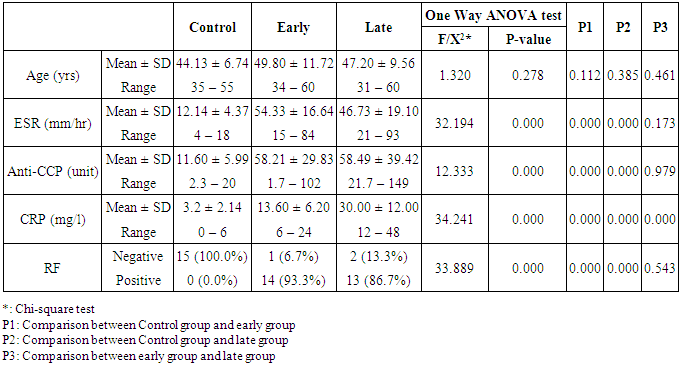
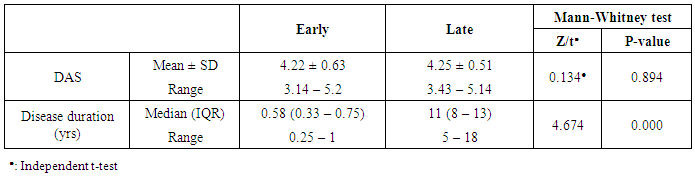
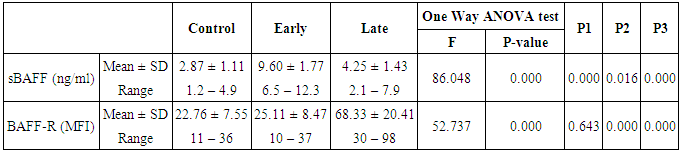
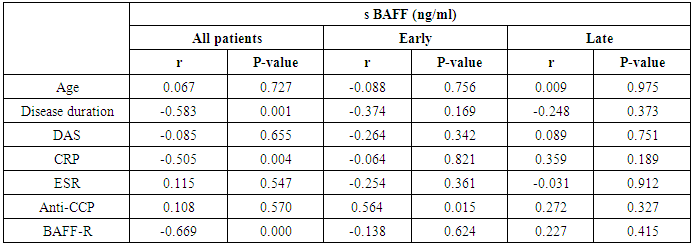
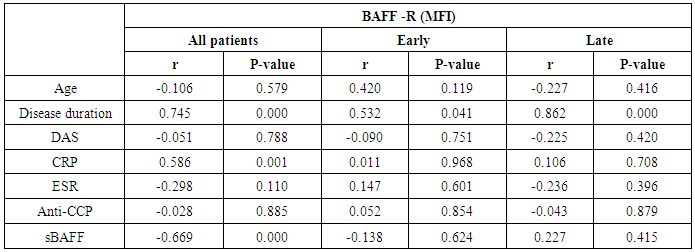
- The results of this study showed a highly significant increase in sBAAF levels in patient group when compared with control (P=0.000). Also, there was a highly significant increase in BAFF-R expression in patient group as compared with control (P=0.002) (Table 2). As regards patient subgroups, we found a highly significant increase in sBAFF levels in ERA patients when compared with control (P1=0.000) and LRA patients (P3=0.000) and a significant increase in sBAFF levels in LRA patients as compared with control (P2=0.016) (Table 5).Also, there was a highly significant increase in BAFF-R expression in LRA patients when compared with control (P2=0.000) and ERA patients (P3=0.000) with no significant difference in BAFFR expression in ERA patients when compared with control (P1=0.643). There was a negative correlation between sBAFF and disease duration (r= -0.583, P=0.001), CRP (r= -0.505, P=0.004) and BAFF-R (r = -0.669, P=0.000) in the patient group (Table 6), while there was a positive correlation between BAFF-R and disease duration (r= 0.745, P=0.000), CRP (r= 0.586, P=0.001) in the patient group (Table 7). In ERA patients, there was a positive correlation between sBAFF and Anti-CCP (r= 0.564, P=0.015) (Table 6),
|
|
|
|
|
|
|
|
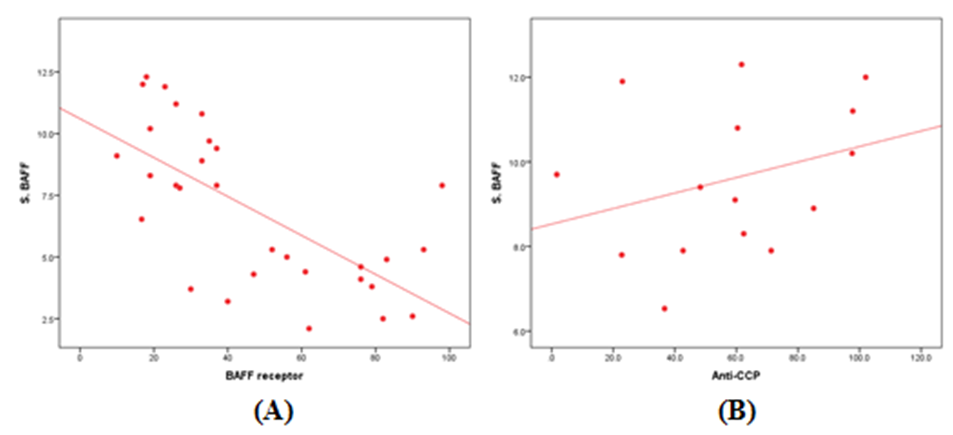 | Figure (1). Correlation of sBAFF with (A) BAFF-R in all patients and (B) Anti-CCP in ERA patients group |
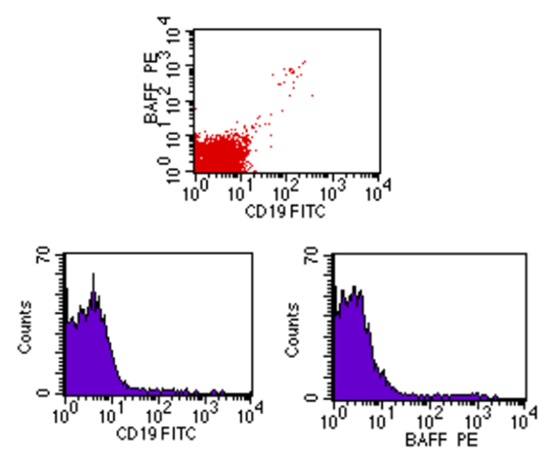 | Figure (2). Flow cytometric analysis of BAFF-R in control showing dim co-expression |
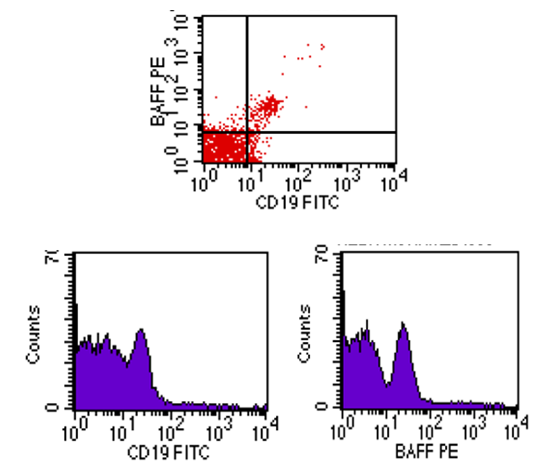 | Figure (3). Flow cytometric analysis of BAFF-R in ERA patients showing dim co-expression |
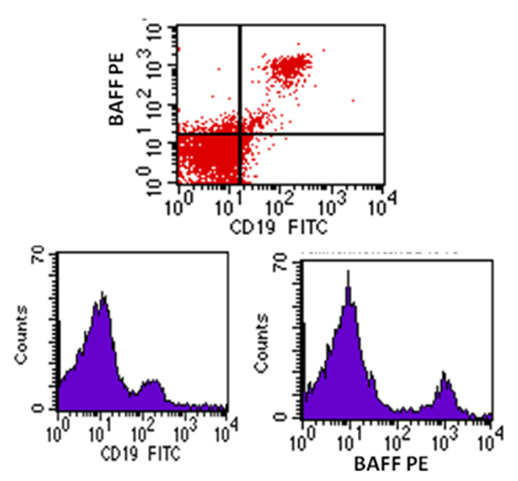 | Figure (4). Flow cytometric analysis of BAFF-R in LRA patients showing high co-expression |
5. Discussion
- Autoimmune rheumatic disorders have complex etiopathogenetic mechanisms in which B cells play a central role [12]. It is widely held that RA is not caused by the dysregulation of a single cell population, but is instead a multifactorial disease involving inappropriate actions and interactions between the cells of the innate and the adaptive immune systems. Cytokines are the major mediator in the most cell-cell interactions between immune cells [13].The B cell activating factor (BAFF) has attracted attention as a potent cytokine, involved in B cell stimulation and survival of autoimmune cells, which makes its pathway a prime therapeutic target for various autoimmune diseases such as RA. Despite, its significance in the pathogenesis of autoimmune diseases, data is limited and inconclusive regarding its expression in different stages of RA [14, 15].In the present study, we found a highly significant increase in sBAFF levels in patients with RA when compared with healthy control, this is in agreement with Morais et al. [12], who demonstrated an increase in sBAFF levels in RA patients and concluded that it plays an important role in B cell survival on the periphery by triggering the B cell survival, differentiation, proliferation and antibody production, which may influence RA autoimmunity. Our results also are in consistent with Šenolt et al. [16], who found that sBAFF levels were significantly higher in patients with RA compared with healthy control. BAFF-R is found on all peripheral B cells. It is considered to be the predominant receptor for B cells. Binding of BAFF to BAFF-R activates the classical and the noncanonical NF-kB signaling pathways, resulting in the expression of a series of downstream genes that are essential for B cell survival [17]. As regards BAFF-R expression, we found a highly significant increase in RA patients when compared with control.In contrary, Gaugler et al. [18] found that circulating BAFF levels and (BAFF-R) expression did not differ between RA patients and healthy control, and the discrepancies between the two studies may be explained by the patients characteristics (age, disease duration), the disease activity and presumably, by the treatment they received. When we compared ERA with LRA patients, we found a highly significant increase in sBAFF levels in patients with ERA as compared with LRA and healthy control, this is in accordance with Moura et al. [19], who found that ERA patients have higher levels of BAFF as compared with established RA and control. They concluded that, the increased levels of sBAFF in ERA patients suggests that, the B cell activation and the development of autoreactive B cell response might be crucial in early phases of RA. Therefore, BAFF could be promising targets for therapy in the early phase of RA. Our results also matched with Bosello et al. [20], who found that BAFF levels were higher in ERA when compared with longstanding RA and healthy control. Gottenberg et al. [21] found that, there is an increase in sBAFF levels in ERA patients when compared with healthy contol. They demonstrated that B cell activation not only depend on the quantity of BAFF secreted and detected in the serum, but also might be related to other mechanisms. Among these mechanisms; membrane bound BAFF or post transitional changes of BAFF (Glycosylation, trimerization with a proliferation inducing ligand (APRIL) or delta BAFF) might affect serum B cell activation.Our results also showed a highly significant increase in BAFF-R expression on B lymphocytes of LRA compared with ERA patients, this is matched with Leandro and Cambridge [4], who found an increased expression of BAFF-R in established RA when compared with ERA. They hypothesized that these changes may occur in response to increased availability of sBAFF and could be a protective response against excessive BAFF stimulation of autoreactive B cells through BAFF-R in the early stage of the disease. This result was also explained by Moura et al. [19], who demonstrated that BAFF increases the chemokine (C-X-C motif) ligand 13 (CXCL13) dependent chemotaxis of memory B cells through BAFF-R triggering. Therefore, increased BAFF level in E RA could support the migration of pre-switch memory B cells towards RA synovium, thus justifying the decrease of this B cell subpopulation in circulation. Increased BAFF-R expression in patients with established RA could be interpreted as representing an increased turnover of BAFF-R due to an increased proportion of activated B cells as disease progress [22].Our results are also matched with Moura et al. [23], who found an increase in the expression of BAFF-R with disease progression. They concluded that disturbances in the expression of B cell related activation and survival factors, particularly BAFF occur from the onset of RA and preceed changes in BAFF-R. These alterations can lead to the development of autoreactive B cells from the first weeks of RA onset.In our study, we found significant positive correlation between sBAFF and Anti-CCP in ERA patients. This is matched with Wei et al. [13], who showed that the increased levels of BAFF results in an increase of autoantibody production e.g Anti-CCP and this is also in accordance with Bosello et al. [20], who found that BAFF levels correlated with Anti-CCP auto antibodies. They concluded that elevated BAFF in ERA patients is related to auto antibody levels and that BAFF levels diminished with treatment along with auto antibody titres suggesting a rational to treat ERA patients with BAFF-targeted agents. Also, we found significant negative correlation between sBAFF and BAFF-R in patients group and this in agreement with Kreuzaler et al. [17], who found that sBAFF levels inversely correlated with the expression of BAFF-R.
6. Conclusions
- The dysregulation of BAFF/BAFF-R system may contribute to the induction and development of RA. Therefore, BAFF/BAFF-R targeting therapy is a promising approach to treat B cell related autoimmune disease e.g rheumatoid arthritis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML