-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2017; 7(2): 40-47
doi:10.5923/j.cmd.20170702.02

Association of Chlamydia pneumoniae Infection in the Pathophysiology of Airway Remodeling in Bronchial Asthma
Ibrahim M. El Akkary1, Mervat E. El Seweify1, Gamal El Din A. El Sawaf2, Gihan A. El Batouti3, Eman Y. Khairy1, Ola A. Salama1
1Department of Clinical Physiology, Medical Research Institute, Alexandria University, Alexandria, Egypt
2Department of Microbiology, Medical Research Institute, Alexandria University, Alexandria, Egypt
3Department of Microbiology and Immunology, Pharos University in Alexandria, Alexandria, Egypt
Correspondence to: Gihan A. El Batouti, Department of Microbiology and Immunology, Pharos University in Alexandria, Alexandria, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background & objectives: Inflammatory tissue damage leading to tissue remodeling has been studied in established chlamydial diseases. Additionally, it has been postulated that Chlamydiapneumoniae (C.pneumoniae) infection may lead to airway remodeling in asthmatics, however, further investigations are needed to define possible mechanisms. The hypothesis that C.pneumoniae infection modulates production of matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), might provide a link between chlamydial infection and airway remodeling in asthma. The present work was designed to study the role of C.pneumoniae infection in the pathophysiology of airway remodeling in bronchial asthma. Methods: Study included 35 asthmatics and 15 normal subjects. Pulmonary flow rates were assessed for all subjects.Methacholine Inhalation Challenge was performed only in asthmatics. Serum samples were tested for detection of C.pneumoniae specific antibodies. Sputum samples were evaluated for levels of MMP-9 and its tissue inhibitor, TIMP-1. Results: Asthmatics demonstrated significant increase in mean level of serum C.pneumoniae IgM compared with control subjects. Significant negative correlation exists between C.pneumoniae IgM and both FVC% and FEV1%, and between C.pneumoniae IgG and both FVC% and FEV1%. No significant correlations were found between C.pneumoniae immunoglobulins (IgA, IgM, and IgG) and either sputum MMP-9, TIMP-1 level or MMP-9/TIMP-1 ratio in both asthmatics and controls. Conclusions: Findings suggest that although there is a decline of lung function related to presence of recent infection of C.pneumoniae, its role in the pathogenesis of airway remodeling in asthma needs further studies.
Keywords: Airway remodelling, Bronchial asthma, Chlamydiapneumoniae, Matrix metalloproteinases
Cite this paper: Ibrahim M. El Akkary, Mervat E. El Seweify, Gamal El Din A. El Sawaf, Gihan A. El Batouti, Eman Y. Khairy, Ola A. Salama, Association of Chlamydia pneumoniae Infection in the Pathophysiology of Airway Remodeling in Bronchial Asthma, Clinical Medicine and Diagnostics, Vol. 7 No. 2, 2017, pp. 40-47. doi: 10.5923/j.cmd.20170702.02.
Article Outline
1. Introduction
- Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyper-responsiveness (AHR) [1]. Many factors contribute to the pathogenesis of asthma, of which respiratory tract organismal infections are considered one of the most important causes of asthma and have been claimed to contribute to the exacerbation of this condition [1, 2]. Among these microorganisms, are C. pneumoniae which is an intracellular gram-negative parasite mainly infecting epithelial cells, endothelial cells and monocytes/macrophages [2, 3]. In contrast to most bacteria, C. pneumoniae must invade cells for replication. Both the intracellular growth and use of host like proteins prevent recognition of C. pneumoniae infections by the host immune system. Thus, C. pneumoniae can be distributed to daughter cells of the originally infected cell, persisting as a slow-spreading latent infection [3]. C. pneumoniae infection has been reported as a possible etiologic agent in asthma since Hahn et al [4] showed an association between C. pneumoniae serology and asthma in 1991. Subsequent studies have demonstrated that C. pneumoniae infection can initiate and exacerbate asthma, this may contribute to chronic asthma symptoms in some patients [5, 6]. The chronic inflammation in asthma is followed by an abnormal healing process that may lead to airway remodeling associated with airway wall thickening, impaired lung function, and abnormal contraction to bronchospastic stimuli [1, 7]. Airway remodeling is characterized by formation of mucus plaques, hyperplasia of myofibroblasts and smooth muscle cells (SMC), and subepithelial fibrosis [7]. Although the mechanisms of remodeling appear to be heterogeneous, an abnormal extracellular matrix (ECM) degradation and deposition may play an important role in the development of structural alterations of the airways, contributing to airway stiffness and irreversible airflow obstruction [7, 8]. Such remodeling within the airway wall is to be attributed mainly to qualitative and quantitative changes of ECM proteins, resulting from an imbalance between proteases and their inhibitors [8].Matrix metalloproteinases (MMPs) are a family of zinc containing endopeptidases. They have an important role in breakdown of ECM components during normal repair processes, including basement membrane collagen, interstitial collagen, fibronectin, and various proteoglycans [9]. Besides, they are considered as biomarkers of tissue damage in several lung diseases [8, 10]. MMPs can be separated into 6 main classes according to their substrate specificity, cellular location and primary structure: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs and others [9]. In particular, MMP-9 (gelatinase B) may play important physiologic roles in lung ECM repair, and in regulating the lung inflammatory response to injury [8]. Furthermore, MMP-9 is thought to be implicated in the pathogenesis of airway inflammatory diseases and is reportedly increased in the airways of asthmatic patients [8, 10].The potent proteolytic activities of MMPs are mainly regulated by their balance with specific tissue inhibitors of metalloproteinases (TIMPs). TIMPs are a family of secretory proteins that are able to inhibit MMPs activities through non-covalent binding of pre- or active forms of MMPs at molar equivalence [9]. By inhibiting MMPs activities, TIMPs are involved in tissue remodeling and regulation of ECM metabolism [10]. TIMPs make up a family of four inhibitors (TIMP-1, -2, -3 and -4). TIMP-1 works as a natural inhibitor of MMP-9 and is found in most tissues and body fluids [9].Deregulation of TIMPs or MMPs activities leads to states of either exaggerated ECM turnover, often leading to remodeling via impaired repair and scar formation, or ECM accumulation leading to fibrosis [8, 10]. Consequently, MMPs and TIMPs and more specially the balance between them may have a crucial role in the pathophysiology of asthma and subsequent airway remodeling [8].Inflammatory tissue damage leading to irreversible scarring and tissue remodeling has been studied extensively in established chlamydial diseases, including trachoma [11], pelvic inflammatory disease [12], and tubal infertility [12], which are caused by one of chlamydial species, Chlamydia trachomatis. Additionally, it has been postulated that C. pneumoniae infection may lead to airway remodeling in a subset of asthmatic patients [13], however, further investigations are needed to define the possible mechanisms. The hypothesis that C. pneumoniae infection modulates the production of MMPs and their inhibitors (TIMPs) [13, 14], might provide a pathogenic link between chlamydial infection and airway remodeling in asthma. The present work was designed to study the role of C. pneumoniae infection in the pathophysiology of airway remodeling in bronchial asthma.
2. Subjects and Methods
2.1. Subjects
- The present study included 35 (6 males, 29 females) stable asthmatic patients as defined by the American Thoracic Society (ATS) [15]. Age, height and weight ranged between 22-45 years, 152- 176 cm and 53-96 Kg respectively. All anti-asthma drugs were withheld 8 hours before the day of the study. All patients were non –smokers and free from pulmonary tract infections. Fifteen normal subjects (3 males, 12 females) were included in the study as a control group. They have comparable age (21-43 years), height (154-190 cm) and weight (49-92 Kg). They were all non-smokers and had never suffered from asthma nor any other allergic disease. Their first-degree relatives were free from atopic disorders. They did not suffer from respiratory tract infection eight weeks prior to the study.
2.2. Study Design
- First day1- Full history taking and examination.2- Pulmonary function tests and if acceptable methacholine inhalational challenge. 3- Blood sampling (serum for serological studies).Second day1- Nasopharyngeal swabs.2- Sputum collection and preparation.
2.3. Methods
2.3.1. Physiological Methods
- Pulmonary Function Tests: Pulmonary flow rates were assessed in asthmatic patients as well as in control subjects. The pulmonary flow rates were measured using computerized dry spirometer (Jaeger from Germany) with automatic dosimeter for methacholine inhalation challenge. They included: forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC % and forced expiratory flow rate between 25% and 75% (FEF25-75%) [16].Methacholine Inhalation Challenge: According to ATS recommendations [17], the five-breath dosimeter protocol was used. The following concentrations of methacholine were prepared in sterile vials: 0.25, 1, 4, 16 mg/ml. The vials were removed from the refrigerator 30 minutes before the test so that the contents warm to room temperature. A nose clip should be applied during inhalation from the nebulizer. The patient was instructed to inhale slowly and deeply from the nebulizer at end tidal exhalation.After 5 inhalations, nebulization stops automatically and FEV1 was measured 2 minutes after end of nebulization. To obtain acceptable quality FEV1, repeated attempts may be required. However, it shouldn’t take more than 3 minutes to perform these maneuvers to keep the cumulative effect of methacholine relatively constant. (The time interval between commencements of two subsequent concentrations should be kept to 5 minutes). At each dose the highest acceptable FEV1 was reported.If the FEV1 had fallen less than 20%, we moved to the next higher concentration and the previous steps starting from nebulization were repeated. When FEV1 had fallen more than 20% from baseline (or the highest concentration has been given), no further methacholine was given, signs and symptoms were noted, and inhaled bronchodilator was administered. The provocational dose of methacholine causing 20% drop in FEV1 (PD20- FEV1) was computed.
2.3.2. Serological Methods
- All serum samples were tested for the detection of C. pneumoniae specific antibodies [IgG, IgA and IgM] by commercially available ELISA kits (R-Biopharm AG, Germany) that was performed according to the manufactures’ instructions [18].Serum samples from all patients and controls were collected and stored at -20°C until the assay time. The frozen samples were slowly brought to room temperature, and mixed gently prior to assay. No grossly haemolysed or lipaemic samples were used. Samples were not subjected to multiple freeze thaw cycles. The sample index was obtained by dividing the absorbance for the sample by the calculated average cut-off control value. Induced Sputum MMP-9 and TIMP-1: Sputum Induction Procedure [19]: Sputum induction was carried out in subjects with a baseline FEV1 > 1.0L. If a subject had FEV1 > 1.0L but < 1.2L sputum induction was performed with normal 0.9% saline. For subjects with FEV1>1.2L, sputum induction was performed with 4.5% hypertonic saline. To prevent contamination of the sputum sample with squamous cells, the subjects were asked to rinse their mouth with water before the procedure. A nose clip was applied and hypertonic saline (4.5%, room temperature) was administered using a mouthpiece and two-way valve connected to a high output ultrasonic nebulizer. Subjects inhaled the saline for 30 seconds first, and then doubling time periods of 1 minute, 2 minutes, 4 minutes. FEV1 was measured at 1 minute after each nebulization period and the percentage fall of FEV1 from baseline was recorded. At each break the subject was asked to clear their throat, cough and transfer any sample into a sterile container. After 15.5 cumulative minutes on the nebulized saline, or at the subject’s request, the induction was stopped. If there was a fall in FEV1 greater than 15% of baseline during the induction, the bronchodilator salbutamol (β2-agonist), was given using a pressurized metered dose inhaler (PMDI). Induced Sputum Processing: All sputum samples were processed as soon as they were collected. Mucus plugs were selected from saliva and dispersed with 0.1% dithiothreitol (DTT). For every mL of sputum, 4mL of DTT was added. The tube was capped and placed on a rotating mixer for 30 minutes at room temperature, to ensure optimal cell dispersion. The supernatant from both portions was aliquoted and stored at –80°C and the frozen samples were brought to room temperature slowly, and mixed.All sputum samples were evaluated for the levels of MMP-9 and its tissue inhibitor, TIMP-1, by respective commercially available ELISA kits (Boster Immunoleaders EK0465, Lithuania) [20]. The ratio of MMP-9 toTIMP-1 in sputum was calculated.
2.3.3. Molecular Detection of C. pneumoniae
- Nasopharyngeal swabs from all asthmatic patients and control subjects were collected by flexible aluminum shafted swabs. C. pneumoniae was detected by using the conventional polymerase chain reaction (PCR) [21].Assay procedure: I. DNA extraction: DNA extraction was performed using Qiagen QIAamp DNA Mini Spin Protocol. ΙΙ. DNA amplification: Reagents used for amplification: 2x PCR master mix (Dream Taq Green PCR master mix) were brought to a final volume of 25µl (each reaction contained 0.05 units/µl Taq DNA polymerase, 2 mM MgCl2 and 0.2 mM dNTPs). Primers: The C. pneumoniae-specific sequences of the PCR primers and probe were selected from the C. pneumoniae (Gen- Bank accession number AF131889) with Primer Express Software (Applied Biosystems, Foster City, Calif.) and synthesized by Applied Biosystems. The PCR product generated (QM85) was 85 bp; and the sequences of the primers were as follows: Forward primer QMOMP1: 5’-GATCCGCTGCTGCAAACTATACT-3’ Reverse primer QMOMP2: 5’GTGAACCACTCTGCATCGTGTAA-3’ Protocol for amplification: Each PCR reaction tube contained 1µl Forward primer, 1µl Reverse primer and 12.5µl DREAM TAQ GREEN PCR master mix. A 10 µl of Qiagen extracted DNA and H2O were added to bring the reaction to a final volume of 25 µl. Thermal profile: Initial denaturation for 95°C for 10 minutes, followed by 40 cycles of PCR amplification, including denaturation for 95°C for 30 seconds, annealing at 58°C for 30 seconds and extension at 72°C for 30 seconds, followed by final elongation at 72°C for 10 minutes. Detection of amplification products: A 2% agarose gel was prepared in TAE (Tris – acetate – EDTA) buffer for detection of positive band with an 85 base pairs. DNA bands were visualized on 302 nm UV trans- illuminator and photographed.
2.3.4. Statistical Analysis
- Data was collected, revised, coded and fed to SPSS version 20. The given graphs were constructed using Microsoft excel software. All statistical analysis was done using two tailed tests and alpha error of value less than or equal to 0.05 is considered to be significant. The following statistical tests were used:A. Descriptive statistics: Included the mean with standard deviation and percent to describe the scale and categorical data, respectively, while median and range were used for skewed data.B. Analysis of numeric data: One-Sample Kolmogorov-Smirnov test, independent samples t-test and Mann-Whitney test. C. Analysis of categorical data: Pearson’s chi square test, Monte Carlo exact test and Fishers exact test. D. Correlation analysis: The correlation coefficient (rho) is expressed as the Pearson coefficient. The sign of the coefficient indicates the nature of relation (positive / negative) while the value indicates the strength of relation as follow: Weak correlation for rho less than 0.25, intermediate correlation for rho of value between 0.25-0.74 and strong correlation for values between 0.75-0.99.
3. Results
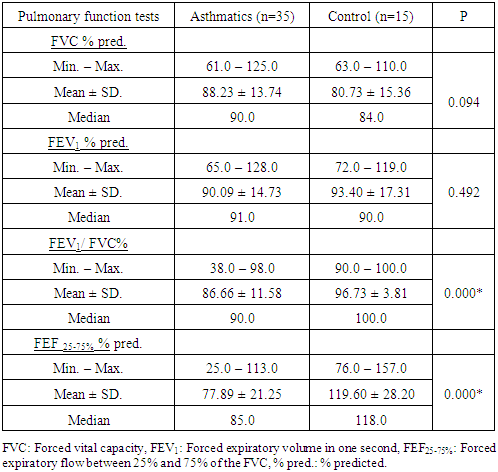
3.1. Pulmonary Function Tests (FVC, FEV1, FEV1/FVC% and the FEF 25-75%)
- There was a significant difference in FEV1/FVC%, and FEF25-75%% pred. between asthmatic patients and control subjects (P = 0.000, and 0.000 respectively) (table 1). No significant difference was found between patients and control subjects in FVC % pred., and FEV1% pred. (table 1).
|
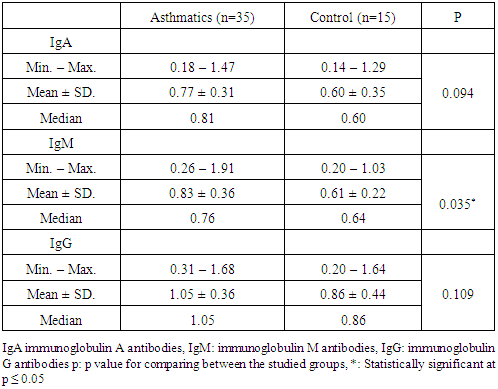
3.2. Serological Tests
- Serological tests performed in this study included the detection of C. pneumoniae specific immunoglobulins; IgA, IgG and IgM.There was a significant difference in IgM between asthmatic patients and control subjects (P = 0.035). As regards, there was no significant difference between patients and control subjects in IgA or IgG (table 2).
|
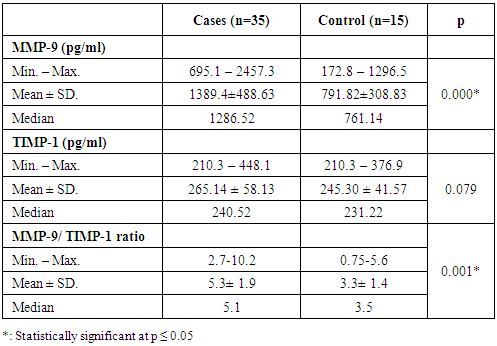
3.3. Sputum MMP-9 and TIMP-1
- There was a significant difference in sputum MMP-9 level between asthmatic patients and control subjects (P = 0.000) (table 3). As regards, there was no significant difference between patients and control subjects in sputum TIMP-1 level.There was a significant difference in MMP-9/ TIMP-1 ratio between asthmatic patients and control subjects (0.001) (table 3).
|
4. Discussion
- Asthma is a chronic inflammatory disorder of the airways that frequently shows progression of airway obstruction. Beside well-defined environmental causes, accumulating evidence suggests that respiratory tract infections play an important role in the pathogenesis of asthma. Among these infections, C. pneumoniae infection has been discussed as possibly inducing the development and exacerbations of asthma [5].The present study shows that recent C. pneumoniae infection is associated with decline in lung functions. Though, the results of the present study provide no direct evidence for an association between airway infection by C. pneumoniae and airway remodeling in asthmatic subjects.The diagnosis of acute C. pneumoniae infection is usually based on serologic criteria that include the presence of IgM antibodies and/or rise in IgG antibodies [21]. The absence of an increase in IgM suggests reinfection rather than primary infection. Reinfection or reactivation of C. pneumoniae infection is followed by elevated IgG antibody levels that persist for months or years, whereas IgA levels decay much more rapidly. For this reason, IgA antibody is considered a more reliable marker for chronic C. pneumoniae infection [22]. In the present study, in agreement with other investigators [4], asthmatic subjects demonstrated a significant increase in the mean level of serum C. pneumoniae IgM compared with control subjects (P=0.035). In addition, C. pneumoniae detection by conventional PCR for asthmatics and control group performed in our study showed negative result for all subjects groups. These outcomes suggested recent infections with C. pneumoniae in our asthmatic subjects. The current study, consistent with previous studies [5, 23], showed no significant difference between asthmatic patients and control subjects as regard C. pneumoniae IgA. Furthermore, in our study, in agreement with other investigators [23], no significant difference was found between asthmatic patients and control subjects in levels of IgG antibodies to C. pneumoniae. This was in contradiction with Agarwal et al., [5]. One possible explanation for our findings may be due to high levels of IgA, and IgG in some of our control subjects, which made it difficult to demonstrate statistically significant differences between asthmatics and control subjects (Of the 15 control subjects, 2 had positive IgA and 5 had positive IgG). These results are confirmed by the estimated data that most people have two or three C. pneumoniae infections during their lifetime [24].To clarify the role of C. pneumoniae infection in bronchial asthma, the relationship between the sample index of C. pneumoniae IgA, IgG and IgM, with FEV1% predicted, FVC% predicted, FEV1/FVC, and FEF25-75%% predicted in asthmatics has been evaluated. In agreement with other investigators [6], we found significant negative correlations between C. pneumoniae IgM and FVC% predicted (r= -0.487, P= 0.003), between C. pneumoniae IgM, and FEV1% predicted (r = -0.395, P=0.019), between C. pneumoniae IgG and FVC% predicted (r = -0.366, P = 0.031) and between C. pneumoniae IgG and FEV1% predicted (r= -0.433, P=0.009). These findings mean that acute C. pneumoniae infection is associated with more declines in pulmonary functions and airway obstruction among asthmatics. On the other hand, we found no significant correlation among asthmatics between C. pneumoniae IgA and any of pulmonary function parameters. This was in consistence with Strachan et al., [22]. This means that acute C. pneumoniae infection can exaggerate asthma in previously asthmatic patients but not initiate asthma in healthy subjects as we found no relation between chronic infection and any decline in lung functions.From a physiological viewpoint, the major features of asthma are bronchial hyper responsiveness (BHR) and variable airway obstruction. BHR leads to airway narrowing in response to various stimuli [1]. In the present study, methacholine challenge testing was used for assessing BHR and provocational doses of methacholine (PD20-FEVı) were estimated. However, no significant correlation was found between BHR, detected by PD20-FEV1, and any of the C. pneumoniae immunoglobulins (IgA, IgG, or IgM). This is consistent with Dejsomritrutai et al., [25] who reported that there is no significant relationship between C. pneumoniae IgG, or IgA with BHR, however, the investigators reported that the seroprevalence of IgM (representing recent infection) was associated with BHR with borderline significance (P=0.05). The ratio of MMP-9 to TIMP-1evaluated in this study is critical as it is considered as the potentially most important member of remodeling markers in asthma [8, 10]. In the present study, asthmatic subjects showed a significant increase in the mean level of sputum MMP-9 and MMP-9/TIMP-1 ratio compared with non-asthmatics (P= 0.000 and 0.001 respectively). Yet, no significant difference could be found in mean levels of sputum TIMP-1 between asthmatics and control subjects. In agreement with our findings, Mohamed et al., [26] reported that MMP-9 and MMP-9/TIMP-1 ratio were increased in both stable asthma group and asthma exacerbation group in comparison to control (P < 0.001), however, during exacerbation, both MMP-9 and MMP-9/TIMP-1 ratio showed significant increase but TIMP-1 did not show significant change when compared to stable asthmatics. These findings suggest that an increase in the serum MMP-9 level may indicate a defect in ECM homeostasis even in stable asthmatic patients. This protease increase may lead to a transient imbalance between MMP-9 and TIMP-1 favoring proteolytic ECM degradation. Conversely, other investigators [27] reported that MMP-9/TIMP-1 ratio in sputum was decreased in the near-fatal attack, severe, moderate and mild asthma groups compared with the control group. Correlations between sputum indices and peripheral airflow obstruction are relevant since induced sputum is considered to originate not only from the central but also from the small airways and alveoli [27, 28]. In this context, the current study evaluated the relation of sputum levels of MMP-9, TIMP-1 and MMP-9/TIMP-1 ratio with parameters of pulmonary function and showed that, there were no significant correlations between any of them in both asthmatics and non-asthmatics. Other investigators [28] showed comparable results with ours. This is in disagreement with other investigators [10]. One possible explanation for the controversy is that our patients may have had milder disease than those in the other study [10].Furthermore, our study demonstrated no significant correlations between sputum MMP-9, TIMP-1 level or MMP-9/ TIMP-1 ratio and PD20-FEV1. This finding is in agreement with Han Z. et al. [29]. On the contrary, Todorova et al., [30] reported that the activity of MMP-9 was positively and strongly correlated with methacholine log PD20. Based on their finding, it is tempting to speculate that MMP-9 may have a beneficial role in asthma. However, one needs to be cautious because only 8 patients were investigated in their study, including only 3 patients with low methacholine log PD20. Therefore, additional studies with more patients across a wider spectrum of asthma severities are warranted. In connection with the suggested role of C. pneumoniae in pathogenesis of asthmatic airway remodeling [14], we have attempted to evaluate the hypothesis that infection with this organism is associated with alterations not only in the secretion of MMP-9 and TIMP-1 individually but also in their ratio. However, in the present work no significant correlations were found between C. pneumoniae immunoglobulins (IgA, IgM and IgG) and either sputum MMP-9, TIMP-1 level or MMP-9/TIMP-1 ratio in both asthmatics and controls.This is in disagreement with Park et al., [14] who investigated whether C. pneumoniae infection affected the secretion of MMP-9 and TIMP-1 by human peripheral blood mononuclear cells (PBMCs) and concluded that C. pneumoniae infection may promote airway remodeling by decreasing the ratio of MMP-9 to TIMP-1 secreted by inflammatory cells. Also, Park et al., [13] concluded that C. pneumoniae-infected human bronchial epithelial cells (BECs) showed enhanced secretion of TIMP-1 compared with non-infected BECs, suggesting that C. pneumoniae may participate in airway remodeling by altering the MMP-9 to TIMP-1 ratio.
5. Conclusions
- In conclusion, our findings suggest that although there is a decline of lung function related to presence of recent infection of C. pneumoniae, its role in the pathogenesis of airway remodeling in asthma needs further studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML