-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2017; 7(1): 18-22
doi:10.5923/j.cmd.20170701.03

Correlation between Serum Glycated Proteins and Iron Deficiency Anemia in Pregnant Females
Aziz A. Abdel-Aziz1, Reham S. Mohamed Ali2, Sahar M. Abdel-Maksoud3, Dalia M. Tawfik4
1Professor Obstetrics & Gynecology Department, Al-Azhar University, Cairo, Egypt
2Assistant Consultant Obstetrics & Gynecology Department, Al-Azhar University, Cairo, Egypt
3Assistant Consultant Clinical Pathology Department, Al-Azhar University, Cairo, Egypt
4M.B.B.CH, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Correspondence to: Reham S. Mohamed Ali, Assistant Consultant Obstetrics & Gynecology Department, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aims: To evaluate the correlation of the serum glycated hemoglobin and serum glycated albumin with the iron metabolism status in non- diabetic pregnant females with iron deficiency anemia. Methods: Fourty pregnant women were subdivided into case and control groups according to the results of their CBC tests done using fully automated cell counter (sysmix-germany). Serum glycated hemoglobin (HbA1c) was measured using The D-10 Hemoglobin A1c Program while serum glycated albumin (GA) was measured by enzyme-linked immune sorbent assay (ELISA). Patients were further stratified according to their gestational age the serum levels were correlated to such stratification. Results: Hemoglobin concentration was lower and HbA1c concentration was significantly higher in late versus early pregnancy in the iron deficiency group. GA did not differ significantly between cases and controls and it showed no significant differences between cases and controls when stratified according to gestational age. Conclusion: Iron deficiency is involved in increased HbA1c levels in late pregnancy. Conversely, serum glycated albumin (GA) may offer a better index for monitoring glycemic control in pregnancy.
Keywords: Glycated haemoglobin, Glycated albumin, Pregnancy, Iron deficiency anemia
Cite this paper: Aziz A. Abdel-Aziz, Reham S. Mohamed Ali, Sahar M. Abdel-Maksoud, Dalia M. Tawfik, Correlation between Serum Glycated Proteins and Iron Deficiency Anemia in Pregnant Females, Clinical Medicine and Diagnostics, Vol. 7 No. 1, 2017, pp. 18-22. doi: 10.5923/j.cmd.20170701.03.
Article Outline
1. Introduction
- Anemia in pregnancy is defined by the World Health Organization (WHO) as a hemoglobin concentration below 11 g/dl [1]. It was further categorized into three levels; mild 9-11 g/dl, moderate 7-9 g/dl and severe < 7 g/dl [2]. Anemia still represents one of the main causes of increased rates of maternal and perinatal mortality, premature delivery, low birth weight and other adverse outcomes [3].Protein glycation is a reaction between protein amino groups and monosaccharides. Since it was reported in vivo, it became an area of interest in the medical studies on diabetes and aging process [4].Glycated hemoglobin, or HbA1c, is the most famous example of glycation in vivo and is used as an index of glycemic control. HbA1c measurements are considerably affected by both erythrocytes turnover and glycemic control. Blood dilution-related anemia and increased demands for iron with associated IDA are frequently observed in late pregnancy. Therefore, HbA1c becomes lower in relation to glycemia in late pregnancy and higher in patients with iron deficiency anemia (IDA) [5].Glycated albumin (GA) is also used as an indicator for glycemic control; but since the half-life of serum albumin is around 17 days, shorter than the life-span of erythrocytes, GA has the advantage to reflect shorter-term glycemic control status over HbA1c. Fuethermore, Serum GA are unaffected by abnormal hemoglobin metabolism [6].No data are present in the literature to show the changes in the concentration of Hb A1c or GA in pregnancy. Therefore, the aim of the present study was to evaluate the correlation of Serum glycated proteins (GA and HbA1c) and iron deficiency anemia in pregnant females.
2. Patients and Methods
- This observational cross-sectional study was held in the Obstetrics and Gynecology Department of Al Zahraa University Hospital. It included 40 pregnant females recruited from the outpatient clinic during antenatal visits. Inclusion criteria: age 20-40 years, gestational age 20-40 weeks, uncomplicated singleton pregnancy. Exclusion criteria: multiple pregnancy, diabetic patients, hypertensive disorders, chronic heart disease or bleeding conditions, women on oral iron therapy.After taking informed oral consent from all patients, and according to Hb level and degree of IDA, patients were subdivided into two groups: Group 1 (control): included 10 women with Hb >10 g/dl.Group 2 (cases): included 30 women with Hb <10 g/dl.The patients were submitted to detailed history taking and general examination to detect symptoms and signs of anemia during pregnancy. Abdominal ultrasound was performed then laboratory workup was carried on as follows:- Blood glucose level (2 hour postprandial) to exclude diabetic patients,- Complete blood Count (CBC); hemoglobin (HGB), hematocrit (HCT), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH) and Mean Corpuscular Concentration (MCHC). CBC was done using fully automated cell counter (sysmix-germany).- Iron profile; serum ferritin, serum iron, Total Iron Binding Capacity (TIBC), Transferrin saturation.- Serum levels of glycated albumin (GA) was detected using enzyme-linked immune sorbent assay (ELISA) based on Biotin double antibody sandwitch technology. Assay range: 0.5 – 200 ng/ml.- Serum HbA1c levels was detected using The D-10 Hemoglobin A1c Program that utilizes principles of ion-exchange high-performance liquid chromatography (HPLC).- Collected data were recorded and statistically analyzed.
3. Results
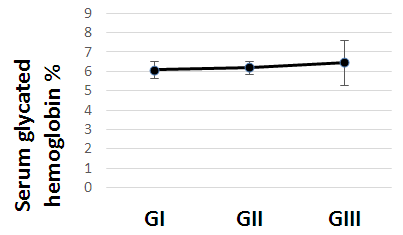 | Figure (1). Serum glycated hemoglobin in all studied cases according to gestational age |
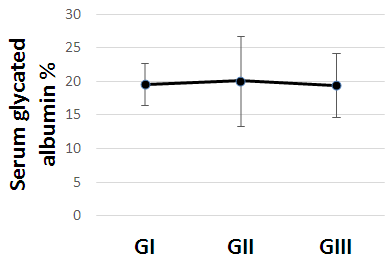 | Figure (2). Serum glycated albumin in all studied cases according to gestational age |
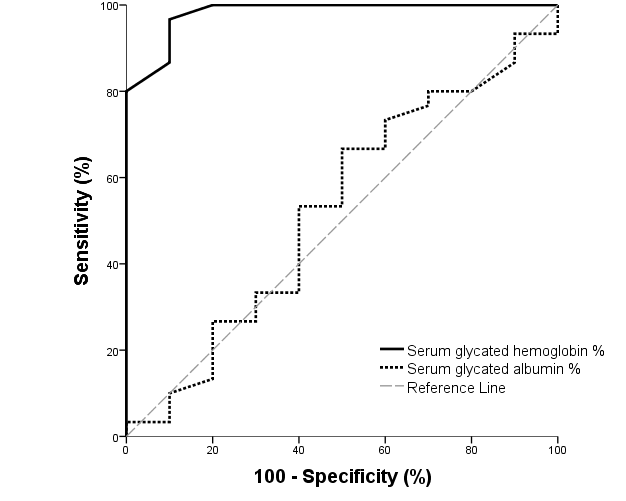 | Figure (3). ROC curve of glycated hemoglobin and glycated albumin for discrimination between pregnant females with IDA and those without |
 | Table (1). Comparison of Serum glycated hemoglobin and albumin between all studied groups |
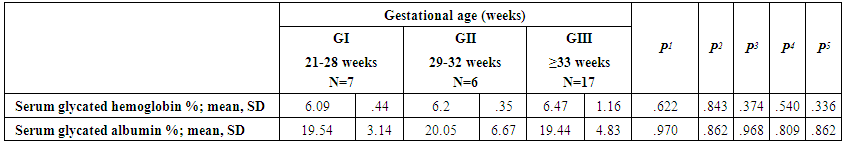 | Table (2). Comparison of glycated proteins between cases according to gestational age |
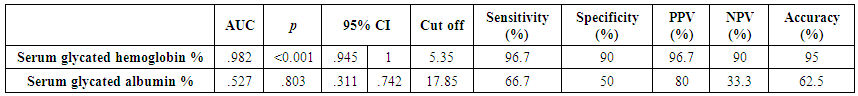 | Table (3). AUC and performance characteristics of glycated hemoglobin and glycated albumin for discrimination between pregnant females with IDA and those without |
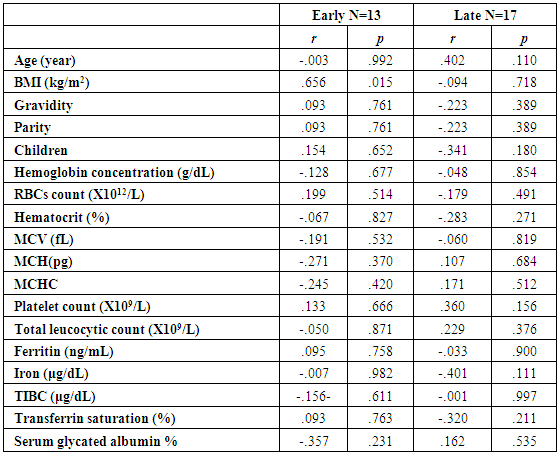 | Table (4). Correlations of glycated hemoglobin with other studied parameters in all studied cases according to gestational age |
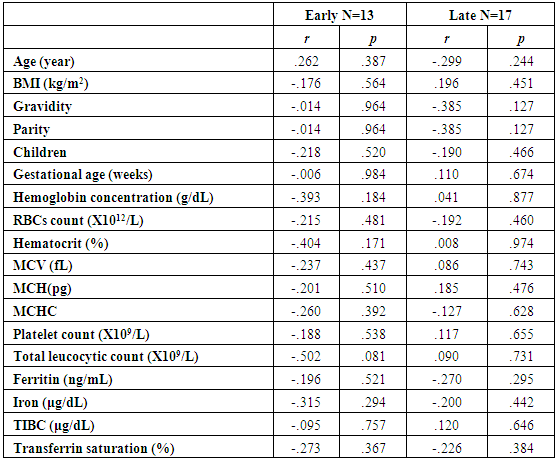 | Table (5). Correlations of glycated albumin with other studied parameters in all studied cases according to gestational age |
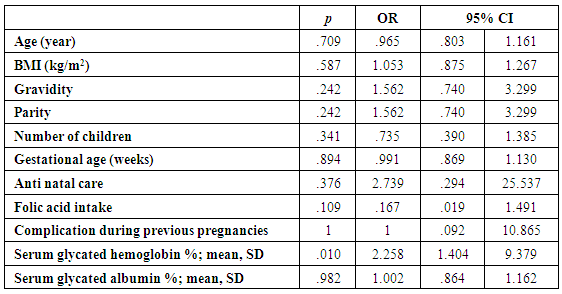 | Table (6). Prediction of IDA in pregnant females |
4. Discussion
- The present study comprised of 30 pregnant females with hemoglobin concentration less than 10 g/dL, and age matched 10 healthy control pregnant females. No significant differences were found between both groups regarding anthropometric measures, education and obstetric history. However, clinical manifestations of IDA were significantly higher and folic acid intake was significantly lower in all studied cases when compared to healthy controls (Table 1).Studied cases showed significantly lower hemoglobin concentration, RBCs count, hematocrit, MCV, MCH, ferritin, iron and transferrin saturation, as well as significantly lower TIBC when compared to healthy controls. Serum glycated hemoglobin showed significantly higher concentration in studied cases when compared to healthy controls. While, serum glycated albumin did not differ significantly between both groups. All studied cases were stratified according to gestational age into GI (21-28 weeks), GII (29-32 weeks) and GIII (≥33 weeks). GI+GII were considered as early pregnancy and GIII was considered as late pregnancy. No significant differences were found in baseline features according to gestational age (Table 2).In the present study, serum HbA1c showed significantly higher concentrations in the studied cases when compared to controls (mean ± SD: 6.33 ± 0.92% and 4.95 0.41% respectively; P<0.001) (Table 1).When stratified according to gestational age, Hemoglobin concentration was lower and HbA1c concentration was significantly higher in late versus early pregnancy (Table 2). MCH & MCHC differed significantly between cases according to gestational age; these differences were attributed to significantly lower MCH and MCHC in GIII versus GI, GIII versus GII and late versus early pregnancy. Otherwise no significant differences were found regarding laboratory data according to gestational age. This was in agreement with the results by [7], who demonstrated that HbA1c levels were lower early in pregnancy and further increased in late pregnancy compared to those in non-pregnant women. They attributed this to iron deficiency that occurs in late stage of gestation and is known to influence HbA1c levels.Conversely, Rai et al. [8], did not observe a significant alteration in HbA1c. However, the mean values in different periods of gestation indicated a biphasic pattern with the lowest values around 24 weeks. This biphasic pattern of decreased HbA1c was also reported by Worth and associates [9], and Phleps and co-workers [10]. They attributed this to influx of new young erythrocytes is nearly half that of the old cells. The influx of new cells in the early phase of pregnancy can be expected to decrease the level of HbA1c. However, further non-enzymatic glycation could lead to an increase in the concentration, which gives an impression of apparent increased rate of glycosylation in later phase of pregnancy.These results were contradictory to a Japanese study by Hashimoto et al. [11], who found that in their population of patients, HbA1c increased early in pregnancy with further increase in late pregnancy. They explained this different behavior by a difference in iron supplementation and metabolism in Japanese women giving these different results.As regards glycated albumin (GA), the present study demonstrated that it did not differ significantly between cases and controls (mean + SD: 19.59 + 5.02% and 19.55 + 4.47% respectively) (P = 0.982) (Table 1). There were also no significant differences between cases and controls when stratified according to gestational age (Table 2).This was in agreement with Koga et al. [6], who found that serum GA levels were unaltered during the pregnancy period. Furthermore, they demonstrated that serum GA levels were unaffected by abnormal hemoglobin metabolism. Therefore, and considering the fact that the half-life of GA is only 17 days, shorter than the lifespan of erythrocytes, they suggested that serum GA represent a better reflection of short-term glycemic control in pregnant females.The results of the present study were different from those of Rai et al. [8], who showed a progressive increase of glycated albumin during normal pregnancy. They also suggested that GA reflects the decrease glucose tolerance that occurs late in pregnancy better than HbA1c levels.In the present study, ROC curve of glycated hemoglobin and glycated albumin was constructed for discrimination between pregnant females with IDA and those without. Glycated hemoglobin showed excellent AUC (AUC=.982, p<0.001) with cut off value = 5.35% for discrimination between pregnant females with IDA and those without. On the other hand, glycated albumin showed poor AUC. Other performance characteristics are shown in table (3).In the present study, BMI significantly showed positive correlation with glycated hemoglobin in early pregnancy. Otherwise, no significant correlations were found between glycated albumin with other studied parameters in all studied cases according to gestational age (Table 4). There was no significant correlations between glycated albumin with other studied parameters in all studied cases according to gestational age (Table 5).The current results are in agreement with those by Takahashi et al. [4], who found significant interrelation between HbA1c and GA in individual cases of pregnancy. But when the data were analysed trimester-wise, the correlation was poor which could be a reflection of the limited number of cases in each group.As regards prediction of IDA in pregnant females, this was done in the present study using age, BMI, gravidity, parity, number of children, gestational age, ANC, folic acid intake, complications during previous pregnancies, serum glycated proteins as covariates. Only higher glycated hemoglobin was considered as risk factor for prediction of IDA in pregnant females (Table 6).These final results are totally in agreement with Hashimoto et al. [11], whose work was the first to demonstrate the involvement of iron deficiency in increased HbA1c in late pregnancy.
5. Conclusions
- The present study revealed that iron deficiency is involved in increased HbA1c levels in late pregnancy. Therefore, caution is warranted in interpretation of HbA1c in late pregnancy. Conversely, serum glycated albumin (GA) may offer a better index for monitoring glycemic control in pregnancy.
Limitations
- The present was performed on a limited number of pregnant women without gestational diabetes mellitus. Further studies on pregnant women with diabetes will represent a better observation that could prove useful for clinical management of these patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML