Amany M. Elsaeed1, Sahar Mohamed Ismail2, Naglaa A. Elgendy3
1Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3Department of Tropical Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Amany M. Elsaeed, Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Early diagnosis of liver cirrhosis in chronic hepatitis C patients is important because cirrhosis per se is an independent predictor of mortality. Cirrhosis is diagnosed clinically, the use of laboratory and imaging data, and liver biopsy. However, these methods have their limitations. These findings suggest the need for diagnostic biomarkers that could add to the sensitivity and accuracy. Serum Kallistatin and cholinesterase may fall into this category of bio markers as the liver represents the major site of synthesis and secretion of both of them and their levels reflect the synthetic function of liver. Objective: To assess serum Kallistatin and cholinesterase levels in cases of liver cirrhosis due to hepatitis C viral infection and correlates their levels with compensated liver cirrhosis and decompensated cirrhosis, and their serum levels with incidence of hepatocellular carcinoma. Methods: Forty-five patients who were included and classified into 15 patients with compensated liver cirrhosis, 15 patients with decompensated liver cirrhosis and 15 patients with hepatocellular carcinoma. They were compared with 15 sex and age matched healthy individuals for control. Levels of serum kallistatin was measured by enzyme-linked immunosorbent assay and serum cholinesterase was measured using colorimetric assay. Results: There was a highly significant decrease in serum Kallistatin and cholinesterase in patients with compensated cirrhosis when compared with control (P<0.000) and decreased levels in decompensated cirrhotic patients. The lowest levels of serum kallistatin and cholinesterase were found in decompensated cirrhotic patients as compared to compensated and HCC patients (P <0.000- P <0.000), respectively. Conclusion: Both serum kallistatin and cholinesterase can be used as reliable biomarkers for the diagnosis of, evaluation of the extent of liver cirrhosis and progression to hepatocellular carcinoma.
Keywords:
Chronic hepatitis C, Kallistatin, Cholinesterase, Cirrhosis, Hepatocellular carcinoma
Cite this paper: Amany M. Elsaeed, Sahar Mohamed Ismail, Naglaa A. Elgendy, Serum Kallistatin and Cholinesterase as Biomarkers for the Diagnosis of Liver Cirrhosis in Patients with Hepatitis C Viral Infection, Clinical Medicine and Diagnostics, Vol. 6 No. 6, 2016, pp. 143-152. doi: 10.5923/j.cmd.20160606.03.
1. Introduction
Liver cirrhosis (LC) is the 14th most common cause of death all over the world that has a high morbidity and mortality. The prevalence of liver cirrhosis is underestimated, because patients at the early phase of liver cirrhosis are often asymptomatic, and most of patients with liver cirrhosis are admitted to hospital due to its related complications [1].The majority of patients with LC die from life-threatening complications, which occurred relatively early in the course of the disease. Thus, the early diagnosis of LC is important for patients with chronic liver disease [2].Liver biopsy remains the gold standard method for the diagnosis of liver fibrosis/cirrhosis [3], and the main disadvantages of liver biopsy are that it is an invasive method, with risk of bleeding, with a mortality rate of 1 in 10,000 [4] and the interpretation of biopsy findings have significant variability in 20% of cases [5]. Also, liver biopsy has the possibility of sampling error which can vary between 33% and 50% as it represents only a small part of the liver [6]. Besides that, liver biopsy increases the economic costs of those patients [7]. For these reasons, many researchers are trying to replace the liver biopsies with noninvasive methods such as serum biomarkers [3]. Moreover, Child–Pugh and MELD (Model of End Stage Liver Diseases) Scores have been widely used to predict the outcomes of cirrhotic patients. However, they have some drawbacks. First, 2 variables (i.e., ascites and hepatic encephalopathy) included in Child–Pugh score are subjective and variable according to the physicians’ judgment and the use of diuretics and lactulose. Second, INR (international normalized ratio), which is one component of both Child-Pugh and MELD scores, does not sufficiently reflect coagulopathy and consequently liver function in liver cirrhosis [8]. Third, there is an inter laboratory variation in INR value [8]. These findings suggest the need to extend the battery of serum markers that could add to the sensitivity and accuracy of currently employed diagnostic and prognostic markers.Kallistatin was first purified and characterized from human plasma as a novel serine proteinase inhibitor (Serpin) and a specific tissue kallikrein inhibitor. Kallistatin has vasodilatory, anti-angiogenic, anti-inflammatory, anti-tumor and anti-oxidant effects. It is present in many human tissues, including the eye, kidney, liver, heart, arteries and veins, blood cells and body fluids. Several studies have shown that the liver represents the major site of synthesis and secretion of kallistatin, which could be a potential biomarker for liver cirrhosis [9].Cholinesterase is an enzyme synthesized by hepatocytes and its serum levels reflect the synthetic function of liver [10]. It catalyzes the hydrolysis of the neurotransmitter acetyl choline into choline and acetic acid; a reaction necessary to allow a cholinergic neuron to return to its resting state after activation [10]. Its activity is reduced in liver dysfunction due to reduced synthesis. The main hepatic source of serum cholinesterase, the marked reduction in its synthesis with hepatocytes dysfunction and restoration of synthesis with hepatocytes recovery proposes that its serum activity might be a more specific indicator of liver dysfunction [11]. Serum cholinesterase is not easily affected by treatment with albumin and blood transfusion in cases of ascites and bleeding tendency which may affect other laboratory markers as serum proteins and blood clotting factors [12].In the light of these data, the aim of this study was to assess serum levels of kallistatin and cholinesterase in cases of LC post hepatitis C viral infection and correlates their serum levels with the progression of cirrhosis from compensated into decompensated and hepatocellular carcinoma (HCC).
2. Patients and Methods
Forty-five patients were recruited from inpatient of Tropical and Internal Medicine departments at Al-Zahraa University Hospital during the period from October 2015 to December 2015 along with 15 ages and sex matched healthy subjects assessed by full clinical history, clinical examination, laboratory investigations and ultrasound examination were allocated to the study as control group. An informed consent was taken from each patient and healthy control after explaining the purpose and implication of the study which was approved by the local ethics committee. The patients were classified into 15 patients with compensated liver cirrhosis, 15 patients with decompensated liver cirrhosis and 15 patients with hepatocellular carcinoma (HCC). The patients were 24 female and 21 male and their age ranged from 45–65 years with mean 53.27 SD± (5.17) years.The patients inclusion criteria were chronic hepatitis C infected patients diagnosed by hepatitis C virus antibodies by ELISA and quantitative polymerase chain reaction and assessed by clinical examination, ultrasound evaluation for signs of liver cirrhosis, decompansation and. Also, chronic hepatitis C infected patients diagnosed HCC by ultrasound examination, alpha-fetoprotein ≥ 400 and triphasic computed tomography (C.T) was included.Exclusion criteria were any chronic liver diseases other than hepatitis C virus infection, history of albumin or blood transfusion four weeks prior to enrolment in the study, history or clinical evidence of variceal bleeding at enrolment. Patients with age <20 or >70 years, or patients with acute infection, chronic malnutrition. Also, pregnant females and those using oral contraceptive pills were excluded from the study.All groups were subjected to the following: A) Careful history taking and thorough Clinical examination, B) Abdominal ultrasonography for all groups, C) Triphasic C.T. for suspected cases of HCC, and D) Laboratory investigations: Liver function tests, Complete blood count, Hepatitis markers (HCV Abs, HBs Ag) using third generation enzyme-linked immunosorbent assay (ELISA) test., Polymerase chain reaction for HCV and HBV to confirm the diagnosis and alpha-feto-protein.Seven mL of venous blood were withdrawn from each subject and divided into two portions: 2 mL of whole blood was collected in evacuated tube containing trisodium citrate as anticoagulant which was sent immediately for the lab for prothrombin concentration measurements which was done on diagnostic stago. The remaining part was centrifuged and serum was separated and divided into two portions. The first portion used for liver function tests assessments which were done on Cobas C 311. The second portion was stored at -20 until be used for cholinesterase and kallistatin measurement. Cholinesterase was measured colorimetrically on biochemistry autoanalyzer Chem 100 (using butyrylthiocholiniodide as substrate) using kit supplied by Centronic GmbH, Germany. Kallistatin was measured by enzyme linked immunosorbant assay (ELISA) technique using kit supplied by Bioassay Technology Laboratory according to manufacture instructions with detection range 0.5-200 ng/ml and sensitivity 0.22 ng/ml [13]. ELISA system used was (Reader A3 1851 & Washer 909) from Das (Italy). ELISA assay employs the quantitative sandwich enzyme immunoassay technique. A monoclonal antibody specific for kallistatin has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any kallistatin present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for kallistatin is added to the wells. Following a wash to remove any unbound antibody–enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Kallistatin bound in the initial step. The color development is stopped and the intensity of the color is measured which reflect the concentration of kallistatin [14].Statistical AnalysisWe calculate sample size according to Raosoft and All statistical calculations were done using SPSS (statistica package for the social science version 22.00) statistical program. at 0.05, 0.01 and 0.001 level of probability [15]. Quantitative data with parametric distribution were done using Analysis of variance. Independent t-test and One Way ANOVA test followed by post hoc analysis using LSD. The confidence interval was set to 95% and the margin of error accepted was set to 5%. The p-value was considered non significant (NS) at the level of > 0.05, significant at the level of <0.05, 0.01 and highly significant at the level of < 0.001. Pearson linear correlation coefficient (r) was estimated to show the relationship between quantitative parameters, Receiver operating characteristic curve (ROC) was used to assess the best cut off point with its sensitivity, specificity, positive and negative predictive values and area under curve (AUC). AUC was interpreted as follow 0.9-1 mean excellent biomarker, 0.8-0.9 good 0.70–0.80 fair and 0.60–0.70 poor. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at the level of < 0.05 [16].
3. Results
3.1. Kallistatin Results
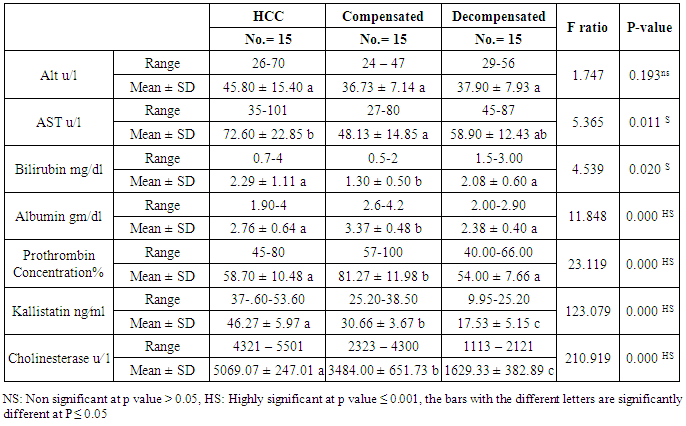
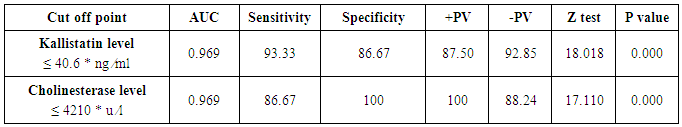
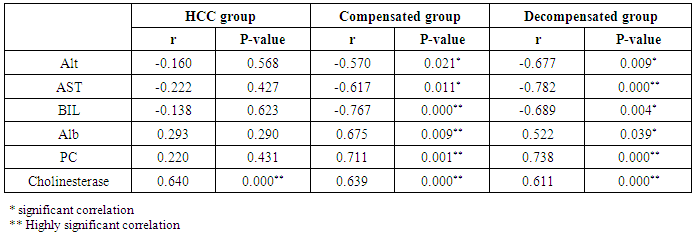
The mean serum kallistatin levels were (30.66 ± 3.67 ng⁄ml), (17.53 ± 5.15 ng⁄ml) and (46.27 ± 5.97 ng⁄ml) in compensated group, decompensated group and HCC group respectively, while it was (89.67 ± 21.99 ng⁄ml) in control group with highly statistically significant decrease in serum kallistatin levels in all patients groups (45 patients) in comparison to control group (15 volunteers) (Tables 1, 2, 3). There was highly significant decrease in kallistatin serum levels in decompensated group (15 patients) in comparison to compensated group (15 patients) (Table 1), while there was highly significant increase in kallistatin serum levels in HCC group (15 patients) in comparison to compensated group (15 patients) and decompensated group (15 patients), respectively (Table 4).Kallistatin serum level at ≤ 40.6 ng ⁄ml (cutoff) can diagnose compensated cirrhosis by 93.33% sensitivity, 86.67% specificity, 87.50% positive predictive value and 92.85% negative predictive value (Table 5, Figure 1).While, kallistatin serum level at ≤ 24 ng⁄ml (cutoff) can diagnose decompensated cirrhosis by 80% sensitivity, 100% specificity, 100% positive predictive value and 83.33% negative predictive value (Table 6, Figure 3).Moreover, kallistatin serum level at ≤ 53.6 ng⁄ml (cutoff) can diagnose hepatocellular carcinoma by 86.67% sensitivity, 93.33% specificity and 92.85% positive predictive value and 87.50% negative predictive value (Table 7, Figure 5).There was a significant negative correlation between serum kallistatin levels and ALT, AST and bilirubin levels respectively while there was a significant positive correlation between serum kallistatin levels and albumin, prothrombin concentration and cholinesterase serum levels respectively in compensated and decompensated groups. On the other hand, there was a highly positive strong correlation between serum kallistatin levels and cholinesterase serum levels in hepatocellular carcinoma, compensated and decompensated group (Table 8).
3.2. Cholinesterase Results
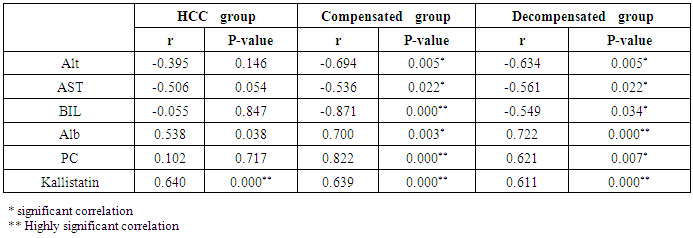
The mean serum cholinesterase levels were (3484.00 ± 651.73 u⁄l), (1629.33 ± 382.89 u⁄l) and (5069.07 ± 247.01 u⁄l) in compensated group, decompensated group and HCC group respectively, while it was (8055.13 ± 1412.65 u⁄l) in control group, with highly statistically significant decrease in serum cholinesterase levels in all patients groups (45 patients) in comparison to control group (15 volunteers) (Tables 1, 2, 3).There was highly significant decrease in cholinesterase serum levels in decompensated group (15 patients) in comparison to compensated group (15 patients) (Table 4), while there was highly significant increase in cholinesterase serum levels in HCC group (15 patients) in comparison to compensated group (15 patients) and decompensated group (15 patients) respectively (Table 4).Cholinesterase serum level at ≤ 4210 u⁄l (cutoff) can diagnose compensated cirrhosis by 86.67% sensitivity, 100% specificity, 100% positive predictive value and 88.24% negative predictive value (Table 5, Figure 2).While, cholinesterase serum level at ≤ 2152 u⁄l (cutoff) can diagnose decompensated cirrhosis by 93.33% sensitivity, 100% specificity, 100% positive predictive value and 93.75% negative predictive value (Table 6, Figure 4).Moreover, cholinesterase serum level at ≤ 5601 u⁄l (cutoff) can diagnose hepatocellular carcinoma by 86.67% sensitivity, 100% specificity and 100% positive predictive value and 69.77% negative predictive value (Table 7, Figure 6).There was a significant negative correlation between cholinesterase serum levels and ALT, AST and bilirubin levels respectively. while there was a significant positive correlation between cholinesterase serum levels and albumin serum levels, prothrombin concentration and Kallistatin serum levels in compensated and decompensated groups, respectively.On the other side, there was a highly positive strong correlation between serum cholinesterase serum levels and Kallistatin serum levels in hepatocellular carcinoma, compensated and decompensated groups (Table 9).Table 1. Results of serum kallistatin, serum cholinesterase levels and liver function tests and their comparison between compensated group and control group
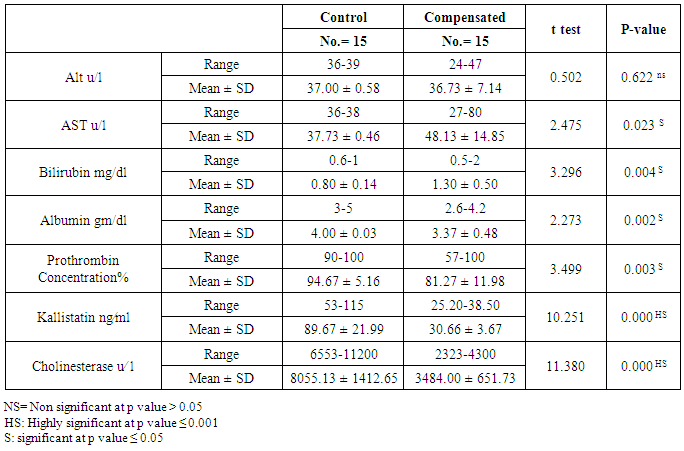 |
| |
|
Table 2. Results of serum kallistatin, serum cholinesterase levels and liver function tests and their comparison between decompensated group and control group
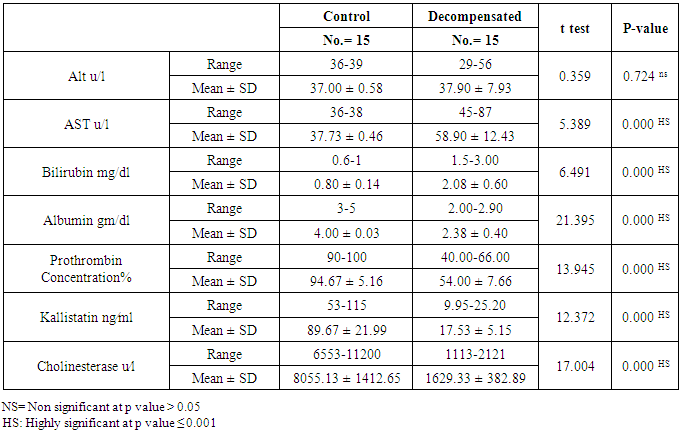 |
| |
|
Table 3. Results of serum kallistatin, serum cholinesterase levels and liver function tests and their comparison between hepatocellular carcinoma group and control group
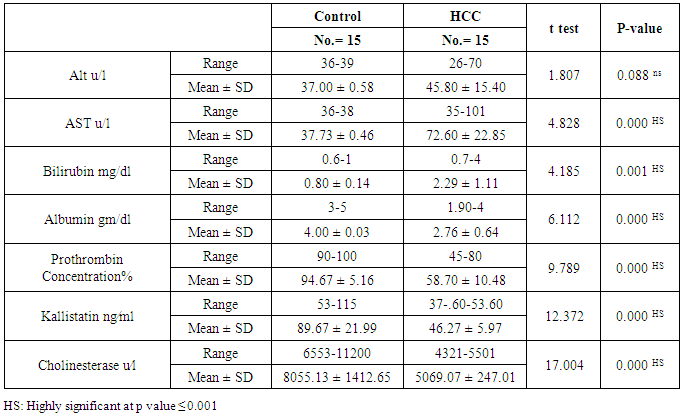 |
| |
|
Table 4. Results of serum kallistatin, serum cholinesterase levels and liver function tests in HCC group in comparison to compensated group and decompensated group
 |
| |
|
Table 5. Serum kallistatin and cholinesterase levels sensitivity and specificity to diagnose compensated cirrhosis and ROC curve
 |
| |
|
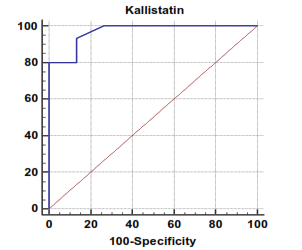 | Figure 1. Serum kallistatin levels can accurately diagnose compensated cirrhosis. The AUC is shown for the performance of the serum kallistatin levels for discriminating compensated cirrhosis from the healthy control groups. The vertical axis represents the sensitivity and the horizontal axis represents the 100-specificity |
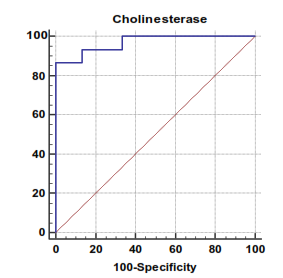 | Figure 2. Serum cholinesterase levels can accurately diagnose compensated cirrhosis. The AUC is shown for the performance of the serum cholinesterase levels for discriminating compensated cirrhosis from the healthy control groups. The vertical axis represents the sensitivity and the horizontal axis represents the 100-specificity |
Table 6. Serum kallistatin and cholinesterase levels sensitivity and specificity to diagnose decompensated cirrhosis and ROC curve
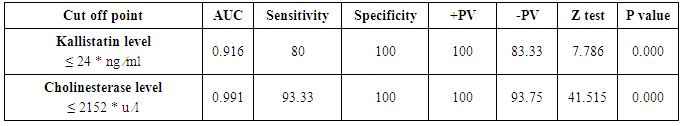 |
| |
|
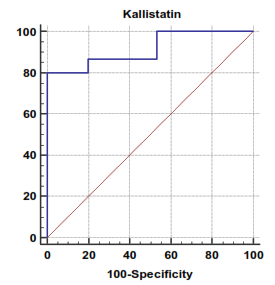 | Figure 3. Serum kallistatin levels can accurately diagnose decompensated cirrhosis. The AUC is shown for the performance of the serum kallistatin levels for discriminating decompensated cirrhosis from the healthy control groups. The vertical axis represents the sensitivity and the horizontal axis represents the 100-specificity |
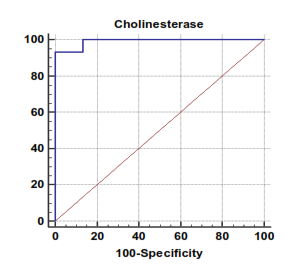 | Figure 4. Serum cholinesterase levels can accurately diagnose decompensated cirrhosis. The AUC is shown for the performance of the serum cholinesterase levels for discriminating decompensated cirrhosis from the healthy control groups. The vertical axis represents the sensitivity and the horizontal axis represents the 100-specificity |
Table 7. Serum kallistatin and cholinesterase levels sensitivity and specificity to diagnose hepatocellular carcinoma and ROC curve
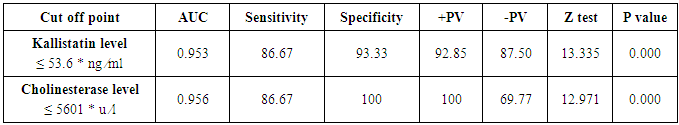 |
| |
|
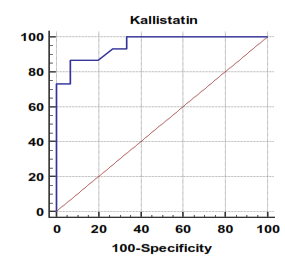 | Figure 5. Serum kallistatin levels can accurately diagnose hepatocellular carcinoma. The AUC is shown for the performance of the serum kallistatin levels for discriminating hepatocellular carcinoma from the healthy control groups. The vertical axis represents the sensitivity and the horizontal axis represents the 100-specificity |
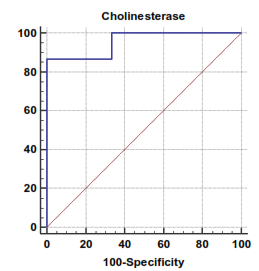 | Figure 6. Serum cholinesterase levels can accurately diagnose hepatocellular carcinoma. The AUC is shown for the performance of the serum cholinesterase levels for discriminating hepatocellular carcinoma from the healthy control groups. The vertical axis represents the sensitivity and the horizontal axis represents the 100-specificity |
Table 8. Correlation of kallistatin and other studied parameters in all patient groups
 |
| |
|
Table 9. Correlation of cholinesterase and other studied parameters in all patient groups
 |
| |
|
4. Discussion
Liver cirrhosis is the most frequent long term consequence of all chronic liver diseases. The annual incidence rates of liver failure (decompansation), HCC, and death in patients with cirrhosis associated with chronic hepatitis B or C infection are approximately 4%, 3% and 3%, respectively [1].Kallistatin, a tissue-kallikrein is present in many human tissues but numerous studies have shown that the liver represents the main site of synthesis and secretion of kallistatin explaining significantly decreased levels of serum kallistatin in patients with LC [9]. In the presented study, kallistatin serum levels were significantly reduced in cirrhotic patient especially decompensated group when compared with normal controls and HCC patients. These results were in consistent with Cheng et al. [9], they showed that serum kallistatin levels in patients with LC were significantly lower than those in healthy controls, demonstrating a close correlation between the reduction in serum kallistatin levels and severity of hepatic disease. They concluded that serum kallistatin levels might therefore offer an additional biomarker for the detection of LC and progressive loss of liver function.Kallistatin is known to play an important role in prevention of cancer, probably through inhibition of tumor growth, anti- angiogenic effects and anti-oxidative effect [9]. Regarding HCC patients in the presented study, serum kallistatin levels were higher in HCC group in comparison to cirrhotic groups (compensated and decompensated patients) but lower than healthy controls. It was expected to found lower levels but not as in cirrhotic patients this could be due to the fact that the human body can up regulate Kallistatin expression when cancers occur in a self- protective mechanism [9].Cheng et al. [9] found an optimal cut-off value for LC diagnosis was 21.64 μg/mL of kallistatin, with sensitivity, specificity, positive predictive value and negative predictive value values were 87.1%, 74.2%, 91% and 62.3%, respectively but with insignificant values in predicting decompansation and hepatocellular carcinoma. On the other hand ,our results had more better predictive values as we found that, Kallistatin serum level ≤ 40.6 ng ⁄ml (cutoff) diagnose compensated cirrhosis by 93.33% sensitivity, 86.67% specificity, 87.50% positive predictive value and 92.85% negative predictive value, while, kallistatin serum level ≤ 24 ng ⁄ml (cutoff) diagnose decompensated cirrhosis 80% sensitivity, 100% specificity, 100% positive predictive value and 83.33% negative predictive value, moreover, kallistatin serum level ≤ 53.6 ng ⁄ml (cutoff) diagnose hepatocellular carcinoma by 86.67% sensitivity, 93.33% specificity and 92.85% positive predictive value and 87.50% negative predictive value.These differences may be related to inclusion criteria of our patients groups as we choose homogenous chronic liver disease patients diagnosed as chronic hepatitis C infected patients and subdivided into compensated, decompensated and hepatocellular carcinoma groups, on the other hand Cheng et al. [9] choose compensated, decompensated and hepatocellular carcinoma groups with different etiologies (viral hepatitis C and B, alcoholic hepatitis and uncertain etiology).Cholinesterase is synthesized in the liver to be transported into the circulation which may provide useful information concerning the state of a cirrhotic patient's liver [10]. In the presented study, serum cholinesterase was significantly reduced in cirrhotic patients especially decompensated group when compared with normal controls and HCC patients. These results in agreement with Meng et al. [11], they reported that serum cholinesterase tended to decrease significantly with the progression of cirrhosis and that the level of cholinesterase was closely correlated with the damage severity of liver cells. They recommended that the combination of cholinesterase with the Child-Pugh score may be more subjective and precise in assessing the liver reserve function of cirrhotic patients.Ramachandran et al. [10], they reported that cholinesterase is an excellent biomarker of cirrhosis with good sensitivity and specificity and a good correlation with liver function tests. They also concluded that cholinesterase distinguishes compensated from decompensated and its low levels in cirrhosis may serve as a useful prognostic marker of advanced liver disease. The presented study showed that kallistatin was correlated with liver function tests in cirrhotic patients and this was previously reported by Cheng et al. [9]. Cholinesterase also correlated with other liver function tests in cirrhotic patients in our study which was in consistent with results reported by Ramachandran et al. [10], they found correlation between cholinesterase activity and serum bilirubin, albumin and INR and also, with Meng et al. [11], where they reported that cholinesterase was correlated with albumin and plasma prothrombin time in the cirrhotic patients, confirming that those substances were synthesized in the liver and reduced in liver dysfunction, due to reduced synthesis.Ramachandran et al. [10] concluded that serum cholinesterase levels below 3506 IU/L had 98.7% sensitivity and 80.3% specificity in predicting cirrhosis. While serum cholinesterase levels less than 2385 IU/L had 80.1% sensitivity and 88.2% specificity in predicting decompensated cirrhosis. Moreover, Gokani et al. [12] reported that difference between the mean serum cholinesterase activity of liver diseases and control group was statistically significant with 90% sensitivity and 100% specificity, suggesting that reduced serum cholinesterase activity strongly indicate liver dysfunctions.Our results showed more better figures as we found that cholinesterase serum level ≤ 4210 u ⁄l (cutoff) can diagnose compensated cirrhosis by 86.67% sensitivity, 100% specificity, 100% positive predictive value and 88.24% negative predictive value. While, cholinesterase serum level ≤ 2152 u⁄l (cutoff) diagnose decompensated cirrhosis by 93.33% sensitivity, 100% specificity, 100% positive predictive value and 93.75% negative predictive value. Moreover, cholinesterase serum level ≤ 5601 u⁄l (cutoff) diagnose hepatocellular carcinoma by 86.67% sensitivity, 100% specificity and 100% positive predictive value and 69.77% negative predictive value.
5. Conclusions
Both serum kallistatin and cholinesterase can be used as reliable biomarkers for the diagnosis, and evaluation of the LC extent as well as progression to hepatocellular carcinoma.
6. Recommendations
Another large study to confirm our results in chronic hepatitis C infected patients. Repeat this study on other different chronic liver diseases.
References
| [1] | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380: 2095-2128. |
| [2] | Benvegnù L, Gios M, Boccato S and Alberti A (2004). Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut, 53(5): 744–749. |
| [3] | Lee UE and Friedman SL (2011). Mechanisms of Hepatic Fibrogenesis. Best Pract Res Clin Gastroenterol, 25(2): 195–206. |
| [4] | Bedossa P and Carrat F (2009). Liver biopsy: the best, not the gold standard. J Hepatol, 50(1): 1-3. |
| [5] | Mehta SH, Lau B, Afdhal NH and Thomas DL (2009). Exceeding the limits of liver histology markers. J Hepatol, 50(1): 36-41. |
| [6] | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC et al., (2009). Liver Biopsy. Hepatology, 49(3): 1017-1044. |
| [7] | Andersen ES, Christensen PB and Weis N (2009). Transient elastography for liver fibrosis diagnosis. Euro J Intern Med, 20(4): 339-342. |
| [8] | Trotter JF, Olson J, Lefkowitz J, Smith AD, Arjal R and Kenison J (2007). Changes in international normalized ratio (INR) and model for end stage liver disease (MELD) based on selection of clinical laboratory. Am J Transplant, 7(6): 1624–1628. |
| [9] | Cheng Z, Lv Y, Pang S, Bai R, Wang M, Lin S, Xu T, Spalding D, Habib N and Xu R (2015). Kallistatin, a new and reliable biomarker for the diagnosis of liver cirrhosis. Acta Pharm Sin B, 5(3): 194–200. |
| [10] | Ramachandran J, Sajith KG, Priya S, Dutta AK and Balasubramanian KA (2014). Serum cholinesterase is an excellent biomarker of liver cirrhosis. Trop Gastroenterol, 35(1):15-20. |
| [11] | Meng F, Yin X, Ma X, Guo X-D, Jin B and Li H (2013). Assessment of the value of serum cholinesterase as a liver function test for cirrhotic patients. Biomedical Reports, 1(2): 265-268. |
| [12] | Gokani R, Jadav P, Shaikh N, Shah R, Chhatriwala M and Patel B (2014). Serum cholinesterase as diagnostic marker of liver disease. International Journal of Biomedical and Advance Research, 5(9): 439-442. |
| [13] | Henry RJ (1974). Cholinesterase. Clin. Chem., Principles and Tech. Harper and Row Publishers Inc., II Edition, 917. |
| [14] | Miao RQ, Agatha J, Chao L and Chao J (2002). Blood, 100: 3245-3352. |
| [15] | Snedecor GM and Cochran WG (1982). Statistical methods-7th edition lowa state Univ., Press, Ames., loww3a, USA., pp. 325-330 |
| [16] | Härdle W and Simar L (2007). Applied Multivariate Statistical Analysis. 2nd ed, Springer, 420pp. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML













