-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(6): 137-142
doi:10.5923/j.cmd.20160606.02

Evaluation of Nucleic Acid Testing for Blood Donors: Four-year Study of the Egyptian Population
Sahar Abd El Maksoud Mohamed, Dalia Mahmoud El Dewi, Dalia Abd El Hafeez Ashour
Assistant Consultant of Clinical Pathology, Lecturer of Clinical Pathology at Al Azhar University, Egypt & Consultant of Transfusion Medicine, Managing Director of Abassia Blood Transfusion Centre, National Blood Transfusion Services, Egypt
Correspondence to: Dalia Mahmoud El Dewi, Assistant Consultant of Clinical Pathology, Lecturer of Clinical Pathology at Al Azhar University, Egypt & Consultant of Transfusion Medicine, Managing Director of Abassia Blood Transfusion Centre, National Blood Transfusion Services, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aims: Blood transfusion is an essential management to save patients life in health care system. Blood is a potential source of transmitted diseases which make the safety of blood products important issue in laboratory medicine. Investigations of transfusion-transmitted infections (TTI), especially hepatitis viruses and acquired immunodeficiency virus, are essential in all blood bank policies. Several methods were applied to screen blood products for hepatitis B (HBV) and hepatitis C (HCV) viruses and acquired immunodeficiency virus (HIV). Methods: Blood donors’ samples for four years were collected and examined for hepatitis viruses and acquired immunodeficiency virus by using serological and nucleic acid testing (NAT). Results: Comparative study showed that NAT is more specific than serologic screening testing for both hepatitis viruses and acquired immunodeficiency virus. However, NAT and serological tests are required to increase the safety of blood components from hepatitis B virus transmission. Conclusion: This study is an attempt to evaluate the effectiveness of introducing NAT for examination of blood components.
Keywords: NAT, HBV, HCV, HIV, NAT, ELISA, ID-NAT, TTI
Cite this paper: Sahar Abd El Maksoud Mohamed, Dalia Mahmoud El Dewi, Dalia Abd El Hafeez Ashour, Evaluation of Nucleic Acid Testing for Blood Donors: Four-year Study of the Egyptian Population, Clinical Medicine and Diagnostics, Vol. 6 No. 6, 2016, pp. 137-142. doi: 10.5923/j.cmd.20160606.02.
Article Outline
1. Introduction
- The safety of blood products is one of the major issues in the area of transfusion medicine. Screening of blood donors for transmissible agents play a major role to decrease the risk of transfusion of infected units. Firstly, testing of antibody/or antigen markers of blood borne pathogens was established. However, limitation of these serological techniques including window period between infection time and detection time, and antigenic variability enhance implementation of NAT. NAT for detection HIV, HCV and HBV has become a routine part of blood donor infectious screening. Infection with HBV and HCV, respectively affects the liver and results in a broad spectrum of disease outcomes. Chronic HBV infection continues to be a major public health issue worldwide [1-4] despite the availability of an effective vaccine and potent antiviral treatments. The risk of developing chronic HBV infection decreases with age at infection, Ninety percent of infected newborns, 30% of children younger than 5 years, and 10% of adults’ progress to chronic infection. [1, 5] 25% of people who acquire HBV as children will develop primary liver cancer or cirrhosis as adults [6].Recent Global Burden of Disease estimates indicate a high morbidity and mortality attributable to chronic HBV, despite decreases over the past decades. [7, 8] Over two billion people throughout the world have been infected with HBV and over 350 million of them are chronically infected carriers and 1. 2 million die from chronic hepatitis, cirrhosis and hepatocellular carcinoma [9]. According to Egyptian studies [10], the prevalence of HBsAg in Egypt is of intermediate endemicity (2–8%). Nearly 2-3 million Egyptians are chronic carriers of HBV. Instead, nucleic acid quantification of hepatitis B virus showed low level viremia in seronegative strains and reliable tools to follow up treated cases [11-14]. The methodology can allow the detection of HBV DNA after HBsAg clearance [15] or detection of HBV lacking serologic markers [16, 17]. However, a relationship between serologic markers and HBV DNA levels has not yet been established.The HCV is a small, enveloped, single-stranded, positive-sense RNA virus. It is a member of the Hepacivirus genus in the family Flaviviridae. There are seven major genotypes of HCV, which are known as genotypes one to seven. [18] The primary route of transmission is intravenous drug use (IDU), blood transfusions and sexual intercourse and other routes [19, 20].HCV affected around 3% of world population. The incidence of HCV infection varies from region to other [21]. Egypt is believed to have the highest rate of hepatitis C in the world (estimated at >10%) [22]. The presence of HCV antibodies (anti-HCV) in patients infected with HCV has led to the development of immunoserological assays that are specific for these antibodies. The presence of anti-HCV antibodies is a measure of prior exposure to HCV infection, but cannot be considered a marker for current infection. In contrast, detection and quantitation of HCV RNA by polymerase chain reaction (PCR) offers a measure of active viremia [23-25]. It is possible by using PCR to detect HCV viremia prior to immunological seroconversion [26, 27] and to detect changes in the viral load in antibody positive, chronic HCV infected and patients undergoing therapy with interferon [28, 29].The HIV is a lentivirus (a subgroup of retrovirus) that causes HIV infection and over time acquired immunodeficiency syndrome (AIDS) [30, 31]. AIDS is a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype [32]. Infection with HIV occurs by the transfer of blood, semen, vaginal fluid, pre-ejaculate, or breast milk. Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells.HIV infects vital cells in the human immune system such as helper T cells (specifically CD4+ T cells), macrophages, and dendritic cells. [33] HIV infection leads to low levels of CD4+ T cells through a number of mechanisms, including pyroptosis of abortively infected T cells, [34] apoptosis of uninfected bystander cells, [35] direct viral killing of infected cells, and killing of infected CD4+ T cells by CD8 cytotoxic lymphocytes that recognize infected cells. [36] When CD4+ T cell numbers decline below a critical level, cell-mediated immunity is lost, and the body becomes progressively more susceptible to opportunistic infections.The main objective of this study is to compare the sensitivity of NAT by using individual test and serological assays by using enzyme immunoassays.
2. Materials and Methods
- Study site and duration of the study This cross sectional study was conducted in Molecular pathology unit (NAT lab), the National Transfusion Bank Center from 2012 to 2015, located in Giza, Egypt. The study group are the donors sample which are send for screening in serological and NAT labs. This study used the archived row data which kept in the lab, the duration of the research was four years between August 2012 and July 2015. After approval was obtained from the manager of the National Blood Transfusion Center before collecting the data.Sample size:The total number of donor samples to be screened is 178685. After extraction of the serum from the participants the following tests are used:1. Serological tests The serology was done using the enzyme linked immunosorbant (ELISA) (hepatitis B surface antigen (HBsAg) by murex HBsAg Kit, hepatitis C virus (HCV) by ortho HCV Kit and HIV by murex HIV kit. 2. Nucleic Acid test (NAT)NAT was introduced in the developed countries in the late 1990s and early 2000s [37]. NAT technique is highly sensitive and specific for the detection of viral nucleic acid, thus helping reduction of the risk of transfusion-transmitted infections (TTIs) The Ulterio assay (Grifols Diagnostics; formerly Novartis Diagnostics) was used for NAT. Simultaneous Multiplex NAT screening of all donations was implemented since 2008. Multiplex NAT yield samples (serology non-reactive samples that are NAT reactive) are further tested using the discriminatory assay to identify the exact viral infection whether HBV, HCV or HIV The individual donation NAT (ID-NAT) assay and not the minipool NAT (MP-NAT) was used in order to ascertain which viral nucleic acid is present in the donor sample. Screening by NAT was done in parallel with ELISA testing for HBsAg, HCV Ab and HIV Ag/ Ab.Transcription Mediated Amplification (TMA) TechniqueTMA uses two primers and two enzymes: RNA polymerase and reverse transcriptase. One of the primers contains a promoter sequence for RNA polymerase.Since each of the DNA templates can make 100-1000 copies of RNA amplicon, this expansion can result in the production of 10 billion amplicons in less than one hour. The entire process is autocatalytic and is performed at a single temperature. Carryover contamination is not a major problem due to the labile nature of the RNA amplicon in the lab environment as well as the use of containment procedures built into the assay procedure.Statistical analysisChi-square (χ2) test was used to measure the association between two qualitative variables. A value of P < 0.05 was considered significant.
3. Results
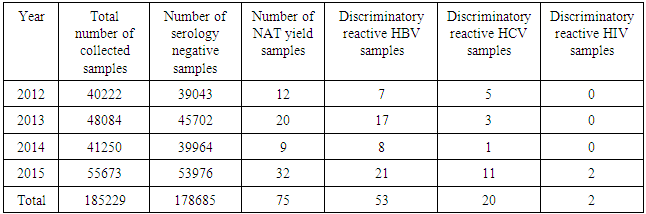
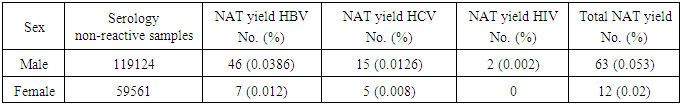
- Analysis of the results of the blood donations collected over 4 years- period from 2012 to 2015 was done. The age of the donors ranged from 18 to 50 years, and they were of both sexes (M: F = 3:1).Table 1 and figure 1 show that NAT screening detected a total of 75 NAT yield donations among 178685 (0.04%) seronegative donations (non-reactive by serology for HBsAg, anti-HCV and anti-HIV-1. Among these 75 NAT yields cases, 53 (0.03%) were reactive for HBV, 20 (0.011%) were reactive for HCV and 2 (0.001%) were reactive for HIV-1.
|
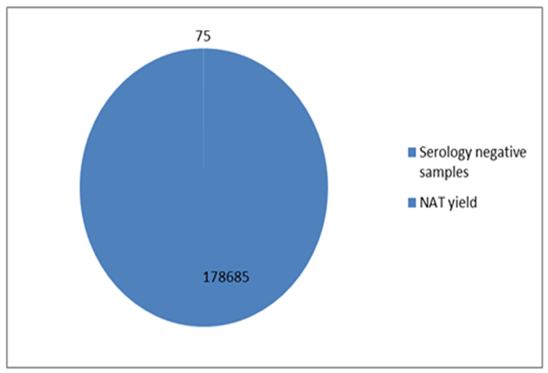 | Figure 1. The NAT yield in 178685 serologically non-reactive samples |
|
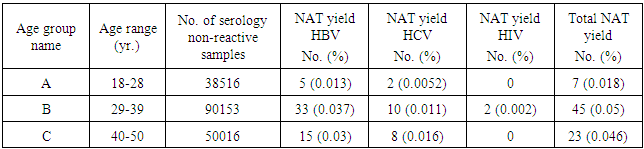
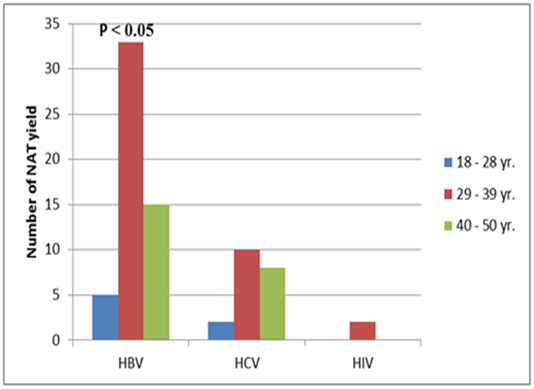 | Figure 2. The NAT HBV, HCV and HIV yields in relation to age |
|
4. Discussion
- Blood safety is a challenge in Egypt because of the high prevalence of HCV and HBV. Nucleic acid amplification test (NAT) technologies have the potential to detect viremia earlier than current screening methods, which are based on seroconversion. NAT detects viral nucleic acids of HIV 1-2, HBV, and HCV at a very low concentration in donor blood. The primary benefit of NAT is the ability to reduce residual risk of infectious WP donations. The estimated reduction of the WP utilizing NAT for HCV is 70-12 days, HIV from 22 to 11 days, and HBV from 59 to 25-30 days. The residual risk for HCV transmission prior to NAT was 0.64/million in France and 3.94/million in Spain which decreased to 0.1/million and 2.33/million, respectively after NAT was adopted. HIV NAT yield rates were estimated at 0.3/million donations in France and Spain as opposed to 0.59 and 2.48, respectively preceding NAT [38].In this study The NAT yield of 75 in 178685 assumes more significance when one considers the fact that single donation is used for generating 3 components that can be used by 3 recipients. Hence, in effect the NAT yield becomes 3 times that is, 225 in 178685. Saving 225 recipients from TTI out of 178685 (0.13%) is indeed very significant. The same percentage (0.038%) was also found in India in 2012 as issued in one review and two original studies [39]. Literature searches did not find similar studies done in Egypt since the introduction of NAT in the beginning of 2009. In a recent cross sectional Egyptian study, the frequency of HBV, HCV and HIV among multi-transfused thalassemic children in Upper Egypt was 4.12%, 37.11% and 0.00%, respectively. The frequency of these serious infections, especially HCV, can be reduced by screening blood donor samples by ID-NAT [40].This fact strengthens the support for the use of NAT despite its cost factors. It means preventing the viral spread of these diseases.
5. Conclusions
- This study shows that the implementation of ID-NAT has a significant impact on the safety of blood supply by allowing the detection of three prevalent viruses that cause serious infections. Effectiveness of nucleic acid testing for blood donors screening is a debating area in transfusion medicine.
ACKNOWLEDGMENTS
- We would like to thank Dr. Afaf Ahmed Ali, Director General of National Blood Transfusion services, Dr. Ghada Attea, Head of Research Department, Abassia Blood Transfusion Center, National Blood Transfusion Services in Egypt, and Dr. Dina Ekram Head of serological department, National Blood Transfusion Services, Minstry of Health, Egypt for their contributions and help they offered us in collecting the data of the present study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML