-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(6): 129-136
doi:10.5923/j.cmd.20160606.01

Evaluate the Sustainability of Viral Response to Antiviral Treatment by IL33 Assessment among Hepatitis C virus Saudi Patients
Nuha Mahmoud Hamdi 1, 2, Haya Salem Al-Jurayb 3, Haifa M. Al-Nafea 4, Roaa Adnan Safar 5, Khulood Mohamed Al Eisa 5, Nagwa Mohamed A. Aref 2, 6
1Clinical Pathology Department, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
2Immunology Departement, Reginal Lab, Ministry of Health, Riyadh, Saudi Arabia (SA)
3Botany and Microbiology Department, College of Science, King Saud University, Saudi Arabia (SA)
4Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Saudi Arabia (SA)
5Reginal Lab. Ministry of Health, Riyadh, Saudi Arabia (SA)
6Microbiology Departement, Ain Shams University, Cairo, Egypt
Correspondence to: Nagwa Mohamed A. Aref , Immunology Departement, Reginal Lab, Ministry of Health, Riyadh, Saudi Arabia (SA).
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In Saudi Arabia, as a result of using blood donation screening tests, the prevalence of CHC has decreased over the last decade. Moreover, the use of polymerase chain reaction (PCR) techniques as a screening tool for blood donors is likely to enhance the decrease in the rate of HCV transmission. Our biomarkers studied included liver function tests compare the HCV viral loads in the serum and six studied interleukins; IL6, IL9, IL10, IL22, IL33 and IFN-γ cytokines in the patient's group.Also, the study included a comparison between the liver function tests with cytokines levels in 57 patients CHC and 31 healthy control (HC). Interleukins showed different response toward CHC infection according to their percentage change concentration in pg/ml of plasma samples. These are as following: IL10 (33.9%), IL33 (30.0%0), IL6 (26.6%), IL9 (25.7%), IL22 (17.0%) and IFN-ɤ (2.4%). IL-33 had a decrease in its level up to 69.72 pg/ml in CHC group after treatment compared to that of HC group with 99.62 pg/ml. IL-33 was thus proposed to function as a novel alarmin (intracellular alarm signal released upon cell injury) to alert the immune system of tissue damage following trauma or infection. Moreover, levels of IL-33 serum correlated with the concentrations of ALT and AST in CHC patients. Plasma ALT and AST level were significantly higher in the CHC patient group than in the control group (54.8±48.1 IU/L vs. 33.4± 27.1 IU/L, p<0.009 and 50.3±45.5 IU/L vs. 27.5± 21.7 IU/L, p<0.002). IL6, IL9, and IL22 levels were found to correlated with treatment resistance to PEG-IFN/RBV therapy. Moreover, viral load had no correlation with patient's gender and age as well as with liver function tests (AST: 0.595, ALT: 0.882, ALP 0.232, DB: 0.362, GGT: 0.326, ALB: 0.829, TB: 0.469). In conclusion, the current study suggests that IL33 can use as an indicator for detecting the response of patients with CHC to combinational treatment to support the results of liver function tests and viral load and to detect the response of patients to therapy and the progression of the disease.
Keywords: Interleukin 33, HCV, Antiviral, Treatment
Cite this paper: Nuha Mahmoud Hamdi , Haya Salem Al-Jurayb , Haifa M. Al-Nafea , Roaa Adnan Safar , Khulood Mohamed Al Eisa , Nagwa Mohamed A. Aref , Evaluate the Sustainability of Viral Response to Antiviral Treatment by IL33 Assessment among Hepatitis C virus Saudi Patients, Clinical Medicine and Diagnostics, Vol. 6 No. 6, 2016, pp. 129-136. doi: 10.5923/j.cmd.20160606.01.
Article Outline
1. Introduction
- Chronic hepatitis C (CHC) is a global problem with a variable prevalence in different countries. It assessed that 140-170 million individuals chronically infected with the hepatitis C virus (HCV), and 3-4 million individuals have infected annually. HCV is one of the leading causes of end-stage liver disease and hepatocellular carcinoma (HCC). HCV transmission is an outcome of blood-borne infection, following the transfusion of infected blood products or the sharing of needles with HCV-infected individuals. The epidemiology of HCV among Arab is unique in its nature and a variety of risk factors associated with its high In prevalence rate [1-3]. A study in King Khalid University Hospital in Riyadh showed a decline in HCV prevalence rates in the blood bank database from 0.58% in 1996 to 0.08% in 2006 [4]. From 2000—2007, the incidence of all three viral hepatitis types HBV, HCV and HAV showed a 20—30% declining trend [5]. The dominant genotype in the KSA is genotype 4, followed by genotype one which is difficult to treat in comparison to genotypes 2 and 3. The combination of pegylated interferon (PEG-INF) and ribavirin for 48 weeks for genotypes 1 and 4 and 24 weeks for genotypes 2 and 3 is the current first-line. PEG-INF is effective in up to 70-95% of patients with genotypes 2 and 3, 44-54% of patients with genotype 1, and 58-86% of patients with genotype 4. The response to such treatment determined after six months by a negative HCV-PCR result which is known as the sustained virologic response (SVR). SVR is affected by other factors such as the gender, viral load and rapid virologic response (RVR), which defined as negative HCV-PCR result after four weeks of therapy.The immune system repression caused by CHC infection and associated damage of hepatocyte [6]. Some evidence suggests that antigen-specific Th1 immunity and pro-inflammatory cytokines play a crucial role in the HCV-related liver injury as well as clearance of viruses. IL-33 may be a pathogenic factor contributing to the CHC-related liver injury [7], the pathogenesis of CHC infection has not yet fully understood [8].The aim of work was to identify biomarkers to predict the outcome of combination therapy in chronic HCV infection and to evaluate liver disease progression and development in CHC patients.
2. Materials and Methods
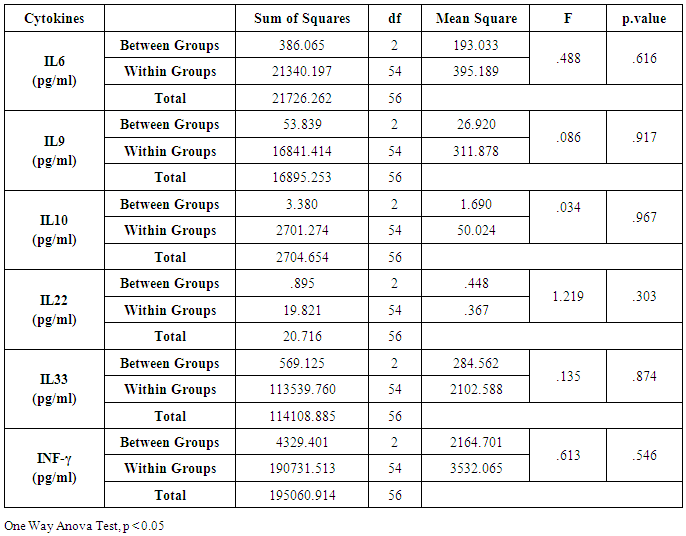
- CHC patients at the Hepatology Clinic at the King Saud Medical City, and Regional lab, Riyadh, Saudi Arabia. The study group included 57 Saudi patients with CHC infection and the second panel is 31 Saudi healthy people (HC). Serum Samples from patients with liver function tests resembles two tubes of isolated serum with the full volume=3ml/tube.MILLIPLEX® MAP Kit [9], EMD Millipore’s MILLIPLEX MAP Human Magnetic Bead Kit is for the simultaneous quantification of the following cytokines: IL-6, IL-9, IL-10, IL-22, IL-33, and IFN-γ.MILLIPLEX MAP is greater multiple technologies offering applications throughout the life sciences. Many kinds of immunoassays can perform on the exterior of MagPlex TM-C microspheres which is a fluorescent-coded magnetic bead. It has color-code microspheres with two fluorescent dyes internal of 100 distinctly colored bead sets. Each sample coated with a specific capture antibody. After the bead captures an analyte test sample, a biotinylated detection antibody introduced. The reaction mixture incubated with Streptavidin-PE conjugate. The microspheres are pliable to pass rapidly through a laser to complete the reaction on the surface of each microsphere that excites. The internal dyes are marking the microsphere set. A second laser is an irritating PE, the fluorescent dye on the reporter molecule. Finally, fast digital signal processors identify each microsphere and quantify the result of its bioassay based on fluorescent reporter signals.Statistical analysisT-Test used for comparisons between cohort groups one-way Anova to find out the significance values between Age and other tests of liver.
3. Results
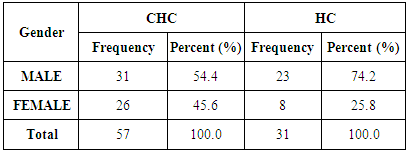
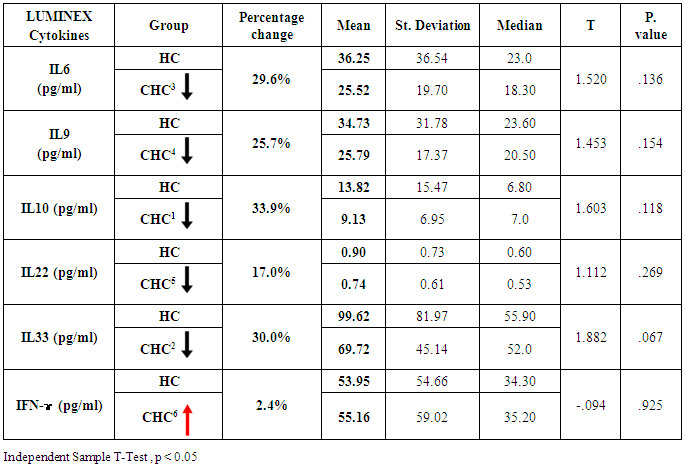
- Demographics percentage data of the patients enrolled showed in Table (1) and Figure (1) related to the allocation of individuals according to the gender variable; their percentage was 54.4% for males and 45.6% for females. The percentage is for HC was 74.2% for men and was 25.8% for women.
|
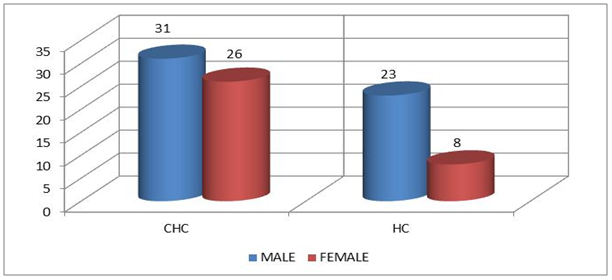 | Figure 1. Distribution of study cohort Saudi individuals according to the gender variable |
|
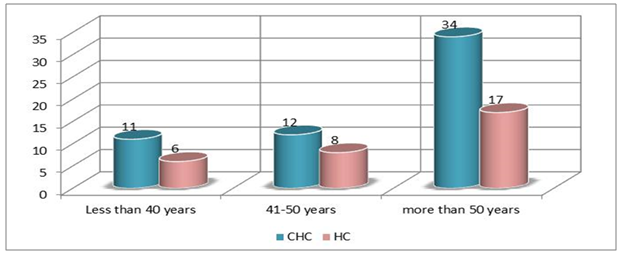 | Figure 2. Distribution of study cohort Saudi individuals according to the age variable |
|
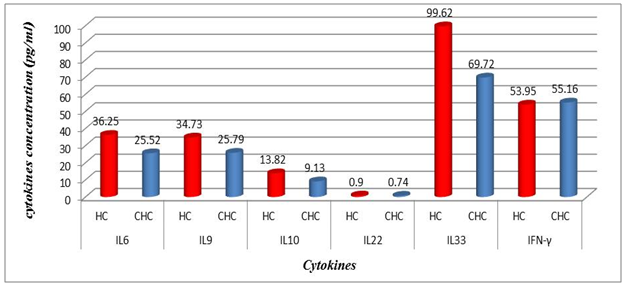 | Figure 3. The average values between treated group (CHC) and Healthy (HC) one according to the studied cytokines results |
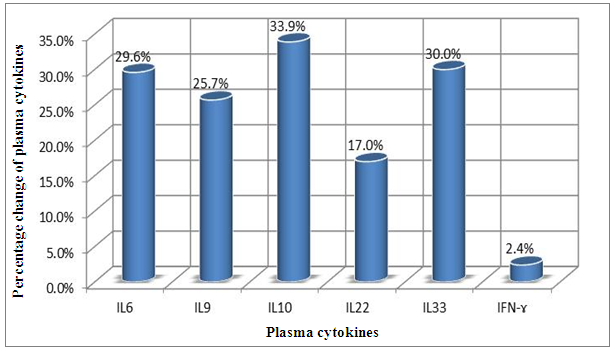 | Figure 4. Percentage change of plasma cytokines concentration in the CHC Patients |
|
|
|
|
4. Discussion
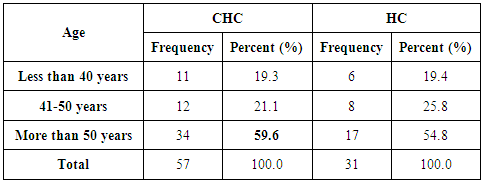
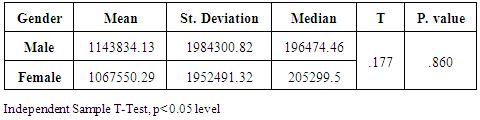
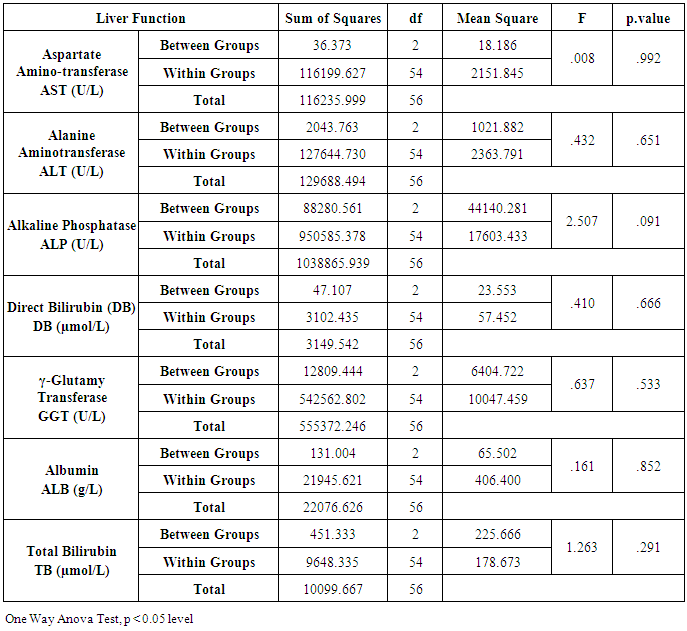
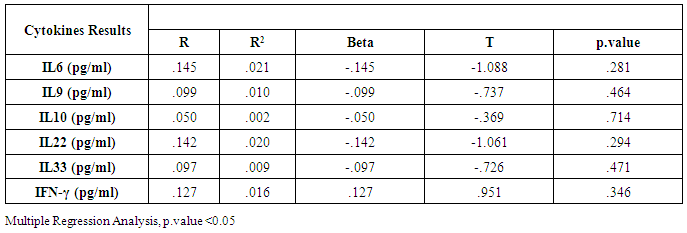
- Chronic liver disease (CLD) characterized by relevant differences in the rate of affected subjects between males and females. The male sex is usually predominant in all except in those conditions that recognize a possible immune basis, like CBP or autoimmune hepatitis [10]. In the present study, the male represented the majority of the CHC frequency was 54.4% in Table (1). Traditionally, the natural history of viral-associate CLD is considered to be more favorable in females, with slower progression to relevant fibrosis and better therapeutic response. Although slow, CLD in women will eventually progress, and cirrhosis and hepatocellular carcinoma (HCC) will develop. Moreover, women and men react differently to combined therapy, especially about the incidence of adverse events and the need for dose modification. Nevertheless, these differences do not influence the SVR rate [11]. In another Asian study, women older than 50 years of age had worse rates of SVR than did men (32% vs. 63%, p = 0.016). By Western studies [12], no differences in SVR shown between gender (p = 0.464), not even among patients older than 50 years of age (40% of women vs. 38% of men; p = 0.859; data not shown). The last data indicated similarity to what we had in Table (3) and for Saudi demographic individuals in CHC under medication Tables (1 and 2).The importance of age about gender underlined when evaluating response to interferon alpha treatment (IFN): women younger than 40 years old had higher rates of SVR than did men (75 % vs. 33 %). Despite the clear-cut protective role of estrogen on the overall clinical condition, there are contradictory data on the effective gain possibly obtained with the addition of hormone replacement therapy [13].In our case study, non-statistically significant differences among the patients according to viral load with the difference of the age variables. Among the three degrees of Viral Load, IU/ml, in patients (high level = 1145703- 8665460, median level= 136209- 799398, and low level = less than 15 -94974) with the difference of the age variable. While gender variables in Table (3); which most male and female categorized between 1143834.13-1067550.29IU/ml. They located in the high-level category. CHC is a serious condition that leads to debilitating liver cirrhosis and fatal hepatocellular carcinoma. Sensitive detection of hepatocellular injury can aid in diagnosis and monitoring of CHC [14]. The main predictors of SVR among Responders to Pegylated Interferon/Ribavirin Therapy in CHC Infected Egyptian Patients had compared to non-responders at baseline before initiation of therapy showed the higher mean of albumin (4.4±0.3 vs. 4.3±0.6gm/dl; p=0.029) [15]. IL-10 can further limit T cells activation and differentiation, leading to suppression of pro-inflammatory responses in tissues [16].It can reasonably argue that the increased levels of IL-10 in patients with CHC reflect the degree of necroinflammation [17]. Contrarily, a reduction of circulating IL-10 levels, reflecting the decrease of transaminases in patients treated with interferon or interferon plus ribavirin, especially in responder subjects, has also been reported by some but not all authors [18]. While, in untreated subjects, increased IL-10 levels are only detectable in those with a more severe inflammatory activity [17]. It concluded that the low IL-10 level before treatment associated with SVR. This cytokine may offer the clinician another tool in predicting treatment outcome of HCV infection [19].IL-33 is likely to be an excellent alarm signal because, due to its constitutive expression in normal tissues, it is ready to be released at any time, for ‘alarming’ ILC2s and other immune cells [20]. IL-33 serum level in CHC patients was significantly higher P < 0.001 than those of spontaneously resolved HCV SR-HCV and HC but decreased after treatment with interferon for 12 weeks [21]. The same results in Table (5) in our study indicated that IL33 is alarmin (alarm signal) following up the medication efficacy according to the liver injury. It was found a decrease in its level up to 69.72 pg/ml in CHC group after treatment compared to that of HC group with 99.62 pg/ml. The concentrations of serum IFN-γ and IL-6 in CHC patients were significantly lower than those of SR-HCV. These data suggest that IL-33 may be a pathogenic factor contributing to the CHC-related liver injury. Apparently, IL-33 is a pathogenic factor, associated with the damage of the liver in CHC patients [21].IL6, IL9, and IL22 correlated with treatment resistance to PEG-IFN/RBV therapy, which is probably one of the most important targets for new treatment strategies for CHC and HCC [22]. Non-responder (NR) patients had higher levels of IL-6, IL-9, IL-10 and tumor necrosis factor (TNF)-a (P 0.05). Plasma levels of IL-6 and IL-9 had a high predictive value for SVR failure [22]. CHC patients in Table (5), were a responder to medication due to the low levels of the following studied cytokines in pg/ml; IL6 (25.52); IL9 (25.79) and IL22 (0.74) compared to HC 36.25, 34.73, 0.90 respectively. High plasma levels of IL-6 and IL-9 had a high predictive value for SVR failure. Clearing of HCV infection associated with low inflammatory and T helper Th2/Th9/Th22 cytokine levels. NR patients had higher levels of IL-6, IL-9, IL-10 and TNF-α in SVR patient [22]. [23] reported that IL-6 up-regulates suppressors of cytokine signaling 3 (SOCS3), a potent inhibitor of the type I IFN pathway, producing IFN-a resistance by suppression of IFN signaling. High plasma levels of IL-6 and IL-9 had a high predictive value for SVR failure. Furthermore, clearing of HCV infection associated with low inflammatory and Th2/Th9/ Th22 cytokines levels.IL-22 mainly produced by Th17, Th22, natural killer (NK) and NK T cells [24]. IL-22 appears to be an important factor in promoting hepatocyte survival and proliferation [25] and provides protection to hepatocytes during acute liver inflammation [26]. SVR patients had reduced levels of IL-22 at week 72 of the follow-up study. Thus IL-22 might play a compensatory role in protecting against hepatocellular damage, and the elimination of HCV might promote a reduction of IL-22, reflecting the stoppage of liver injury [22].IFN-γ levels can aid clinicians further to identify high-risk patients who may fail PegIFN/RBV therapies and enable appropriate decisions for more personalized medicine in these cases [27]. It was evident in Table (5), and Figure (4) that change proportion percentage in IFN-γ resemble the lowest level of concentration (2.4%) compared to IL33 (30%) which illustrated one of the highest levels of all the studied cytokines. Supporting the notion that pro-inflammatory cytokines, such as IFN-γ and IL-6, are not only important factors for the clearance of infected HCV, but also for liver injury [28]. A study demonstrated that IFN-γ could serve as an important biomarker of achieving SVR. Not only the elevated IFN-γ concentrations at specific time points but also the total IFN-γ amounts strongly linked to non-response with PegIFN/RBV treatment. IFN-γ was an independent predictor of SVR as analyzed by the multivariate logistic regression test [27]. In the present study, the analysis of 57 patients with chronic hepatitis C treated with combination therapy demonstrated that HCV load does not correlate with the six studied cytokines in Tables (6 and 7). Viral load implies much more than numbers, and the correct interpretation of these data should consider a broader context depending on multiple factors that are more complicated than the simple value obtained upon quantification [29]. Bioinformatics study further revealed that TNF and IL-10 are the major attributes able to categorize HCV patients aside from non-infected individuals with a global accuracy of 100 %, reaching 86 %, after leave-one-out cross validation response [30]. The activity of pro-inflammatory and regulatory cytokines is a dynamic event that will influence the progression of liver injury, contributing to reducing the damage caused by the virus or regulating the excessive response of response of effector cells [30].
5. Conclusions & Recommendations
- The present study indicated that sustained virological response (SVR) to interferon-based therapy in patients with noncirrhotic CHC eliminates liver-related complications in the long term by combining indicator data of liver function, viral load, proinflammatory and regulatory cytokines.It has a potential pathophysiological role in the persistence and progression of hepatitis. It was demonstrated a decrease in the serum level of IL-10 during treatment from 56 pg/ml as a baseline and remained low in patients 30 pg/ml with SVR. It considered a good predictive noninvasive marker to predict response to interferon and ribavirin therapy in HCV patients. It can aid clinicians to identify further high-risk patients who may fail PegIFN/RBV therapy and allow for the adoption of appropriate strategies for more personalized medicine.IL33 is alarmin (alarm signal) following up the medication efficacy according to the liver injury. It was found a decrease in its level up to 69.72 pg/ml in CHC group after treatment compared to that of HC group with 99.62 pg/ml. It can release into the extracellular space after cell damage (necrotic cell death) or mechanical injury. It was an excellent alarm signal and a pathogenic factor contributing to the CHC-related liver injury.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML