-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(5): 111-121
doi:10.5923/j.cmd.20160605.01

Evaluation of Tumor Necrosis Factor Receptor 1 (TNFR1) in Diagnosis of Ventilator-Associated Pneumonia
Asmaa Abd EL Wakeel El Sehemawy 1, Mona Abd EL Rahman EL Dosoky 2, Hend Ezzat 2, Noha Abdel-Rahman ELdesoky 3
1Department of Pediatrics, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
2Department of Clinical Pathology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
3Department of Biochemistry, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt
Correspondence to: Asmaa Abd EL Wakeel El Sehemawy , Department of Pediatrics, Faculty of Medicine, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2016 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective: Ventilator associated pneumonia (VAP) is defined as nosocomial pneumonia in mechanically ventilated patients. It is considered to be the most important cause of infection-related deaths in the intensive care unit. We studied the characteristics and risk factors of VAP in critical-ill neonates and evaluated Tumor necrosis factor receptor-1(TNFR1) in non-bronchoscopic alveolar lavage (NB-BAL) as a sensitive marker for diagnosis of neonatal VAP. Methods: Sixty neonates admitted to neonatal intensive care unit (NICU), of Al-Zahraa Hospital, Al-Azhar University in the period from April 2015 to February 2016, who needed mechanical ventilation were included in the study. 30 neonates (18 males and 12 females) with proven diagnosis of VAP, together with another 30 neonates (16 males and 14 females) without VAP served as control group. All studied neonates were subjected to perinatal history taking, clinical examination, routine investigations (Complete blood count, C-reactive protein, blood culture and liver and kidney function tests), chest X-ray daily and NB-BAL culture as well as TNFR1assay in NB-BAL. Findings: Of 60 neonates who needed mechanical ventilation, 50% of them developed VAP. Intubation more than three times and umbilical catheter insertion were risk factors for developing VAP. Increased total leucocytic count and CRP were significantly presented in VAP group. There were significant increase in VAP than non-VAP groups regarding vital signs and mechanical setting (Peak inspiratory pressure and vent rate) and decreased O2 sat % after 48 hrs of mechanical ventilation compared to the onset of ventilation. Increased mucopurulent endotracheal tube secretion in VAP group was observed. The most abundant microorganism associated with blood stream and NB-BAL in VAP group was Klebsiella (20% and 56.7%) respectively. There was also significant increase of TNFR1 in NB-BAL in VAP group (36.68 ± 23.27) compared to non VAP (26.98 ± 7.33), (p= 0.036). Conclusions: Intubation more than three times and umbilical catheter insertion were risk factors for developing VAP. TNFR1 in NB-BAL is a sensitive marker for diagnosis of neonatal VAP.
Keywords: Pneumonia, Mechanical ventilation, Newborn, Intubation
Cite this paper: Asmaa Abd EL Wakeel El Sehemawy , Mona Abd EL Rahman EL Dosoky , Hend Ezzat , Noha Abdel-Rahman ELdesoky , Evaluation of Tumor Necrosis Factor Receptor 1 (TNFR1) in Diagnosis of Ventilator-Associated Pneumonia, Clinical Medicine and Diagnostics, Vol. 6 No. 5, 2016, pp. 111-121. doi: 10.5923/j.cmd.20160605.01.
Article Outline
1. Introduction
- The incidence of healthcare-associated infections (HAI) in neonates and paediatric intensive care units (PICUs) is high [1]. This is due to the use of invasive devices such as central vascular lines and mechanical ventilation and thus increase the burden of ventilator-associated pneumonia and other HAI [2].Ventilator-associated pneumonia (VAP) is a serious complication related to mechanical ventilation in the neonatal period and is the most common nosocomial infection in neonatal intensive care unit (NICU) [3]. VAP is defined as nosocomial pneumonia in mechanically ventilated patients that develops more than 48 hours after initiation of mechanical ventilation (MV) [4].Diagnosis of a VAP episode requires a combination of clinical, laboratory and radiological criteria. The most prevalent clinical signs associated with VAP refer to changes in the characteristics and volume of respiratory secretions and the appearance of purulent mucus in tracheal aspirate (TA). Other signs include hypo- or hyperthermia and worsening of the respiratory distress [5].Despite the advancements in antimicrobial regimens, VAP continues to be an important cause of morbidity and mortality. VAP requires a rapid diagnosis and initiation of appropriate antibiotic treatment, to improve the patient's prognosis and decrease the emergence of multidrug-resistant (MDR) pathogens [6].Tumor necrosis factor (TNF) - α is a pleiotropic early response cytokine. TNF-α supports host antibacterial defences through several mechanisms, including potentiation of the killing of bacteria by neutrophils and upregulation of vascular and adhesion molecules, which are necessary for neutrophil recruitment [7]. TNF- α binds to two different membrane receptors, TNF receptor (TNFR) 1 (also known as p55, p60, or CD120a) and TNFR2 (also known as p75, p80, or CD120b). TNFR1, which contains a death domain (DD) within its intracytoplasmic region, is thought to be the key receptor for TNF signalling. TNF-α signalling is finely regulated by the availability of TNFR1 at the cell surface. The cleavage of the receptor ectodomain by ADAM17 culminates TNFR1 signalling and produces a soluble receptor that potentially neutralizes circulating TNF-α [8]. Activation of ADAM17 and shedding of TNFR1 by immune cells have been reported in response to septic stimuli in vitro and in animal models of LPS-induced endotoxemia. Shedding of TNFR1, which occurs between 6 and 24 h after LPS inoculation, was critical for the neutralization of TNF-α signaling and the arrest of inflammation [9].
2. Materials and Methods
- This study was performed between April 2015 to February 2016 at the NICU of Alzhraa University Hospital (Cairo, Egypt). Informed consent was obtained from the participating parents in adherence with the guidelines of the ethical committee of Al-zahraa hospital, AL-Azhar University, Cairo, Egypt.The study included 60 newborns classified into (2) groups according to clinical and laboratory findings. Thirty of them (30) have ventilator Associated Pneumonia (VAP group) based on clinical and laboratory data (12 female and 18 male). And (30) neonates on a mechanical ventilator with no clinical signs or laboratory evidence of pneumonia were taken as control group (non VAP group) (14 female and 16 male).All participants were of gestational age 37-42 weeks, mechanically ventilated for ≥48 h. All patients and controls were subjected to chest X ray, NB-BAL for cytologic and bacteriologic examination and assay of TNFR1 after 48 hours of mechanical ventilation. Blood samples were withdrawn for Blood culture, CBC, CRP. Also monitoring and recording of vital signs and mechanical ventilation settings were done for all patients.
2.1. VAP Diagnosis
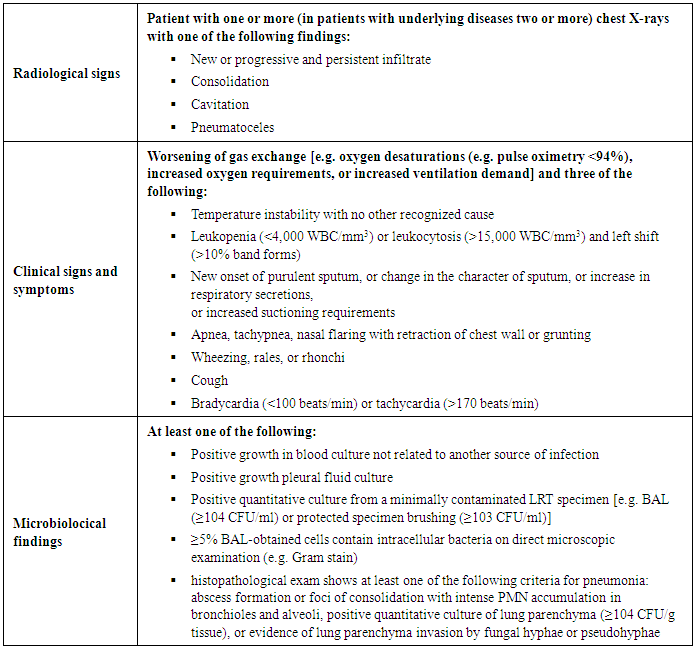
- The Centers for Disease Control and Prevention (CDC; Atlanta, Ga., USA) defines VAP as ‘a nosocomial infection diagnosed in patients undergoing MV for at least 48 h’ [10]. It is noteworthy that diagnosis of a VAP episode requires a combination of radiological, clinical, and laboratory criteria (table 1) [10].
|
3. Sampling Procedure
- 3.1. Two ml were drawn and placed into a vacutainer tube containing EDTA for complete blood picture (CBC) using Kx 21haematology analyzer (Sysmex, Kobe. Japan).3.2. Three ml were drawn and placed into a plain tube, centrifuged within 30 minutes of collection and serum was separated for measuring Alt, Ast, urea, creat (using fully automated chemistry analyzer Cobas Integra 400 plus), Na+ and K+ (using AVL 9180) and CRP (using latex agglutinaion) at the same day.3.3. Collection procedure for Blood culture: site was cleaned with 70% isopropyl alcohol, allowed to air dry, followed with Chlorhexidine Prep using back and forth friction scrub for 30 seconds, allowed to dry for 30 seconds. When required amount of blood was received, needle was removed from skin. Tops of blood culture bottles were removed and cleaned with 70% isopropyl alcohol and allowed to dry. Blood specimens were injected into bottles of blood culture (anaerobic bottle, then the aerobic), labelled and sent to the laboratory. Detection of bacterial growth within 7 days was done by using automated Bact alert system.3.4. Collection of endotracheal tube (ETT) secretion by non bronchoscopic bronchoalveolar lavage: • Proper hand washing and wearing sterile gloves were done.• The technique involves wedging a size 6F or 8F end hole suction catheter. A size 8 suction catheter was used for endotracheal tube size 3.5 mm internal diameter, and size 6 for 3.0 mm or smaller. The infant was positioned supine with the head turned 90° to the left; such a position virtually ensures that a catheter advanced down the trachea enters the right main bronchus. While ventilation continued, the catheter was introduced into the endotracheal tube and gently advanced until met with resistance. An aliquot of 1 ml per kg body weight of saline, warmed to 37°C, was instilled through the catheter, coinciding with a positive pressure inflation of the lung. • Low-pressure suction was then immediately applied (60 to 90 mm Hg for a size 8 catheter, 80 to 120 mm Hg for a size 6), and the return fluid was collected in a mucus trap. A total of three aliquots were instilled, with suction after each. The fluid returned from all aliquots was pooled. • The specimen collected was immediately transported to the laboratory for examination.
4. Cytologic Analysis
- The cytologic study of NB-BAL was performed on a homogeneous sample. This sample was centrifuged and the NB-BAL fluid was stained using Gram’s stains. A total cell count was performed on aliquots of resuspended NB-BAL using a hemocytometer counting chamber. Slides were stained with Gram’s stains. The Gram’s stain was examined at high magnification (x100) to determine the morphologic features of bacteria and the percentage of ICB (intracytoplasmic bacteria within macrophages and neutrophils) by counting 100 cells.
5. Bacteriologic Analysis
- The specimen was cultured on sheep’s blood, chocolate agar, and MacConkey plates and all bacterial species isolated were identified by standard microbiological technique. Each distinct colony was counted separately, multiplied by 10 and recorded as colony forming units per milliliter [(cfu)/ml] of BAL. We used 104 cfu/ml of BAL as the quantitative cut-off point for culture positivity.
6. Tumor Necrosis Factor Receptor-1 Assay
- Enzyme linked immuno sorbent assay (Sandwich ELISA) was used to detect and quantify levels of TNFR1.the kit was provided by glory science company, its catalog number was 15897. A polyclonal antibody specific for TNFR1 has been pre-coated into the microwells. The TNFR1 protein in samples was captured by the coated antibody after incubation. Following extensive washing, a monoclonal antibody specific for TNFR1 was added to detect the captured TNFR1 protein. For signal development, horseradish peroxidase (HRP)-conjugated antibody was added, followed by Tetramethyl-benzidine (TMB) reagent. Solution containing sulfuric acid was used to stop colour development and the colour intensity which is proportional to the quantity of bound protein was measurable at 450nm.The detection range of the kit was 5pg/ml-180pg/ml.
7. Radiological Investigation
- Chest X ray at the onset of MV and after 48 hours of mechanical ventilation was done.
8. Statistical Methods
- Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version20. Quantitative variables were expressed as mean±SD, and comparisons between the two groups of infants (VAP group and non-VAP group) were performed using the unpaired Student’s t-test. Qualitative variables were expressed as observed numbers or proportions, and comparisons between infants with and without VAP were performed using chi-square contingency test. Spearman correlation test was used to assess the relation between two studied parameters in the same group. In all statistical testing procedures, p <0.05 was considered significant. Receiver operating characteristic (ROC) curve was used to assess the best cut off point with sensitivity and specificity.
9. Results
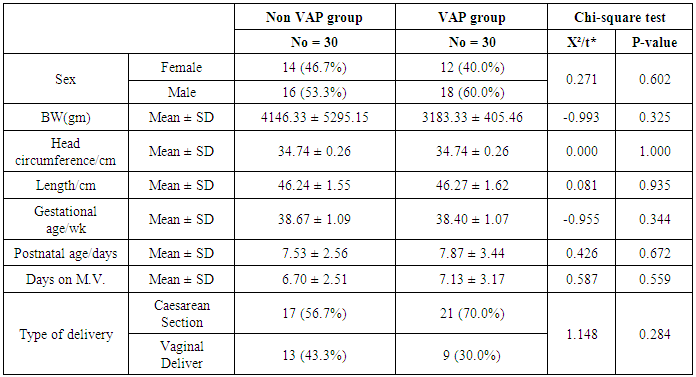
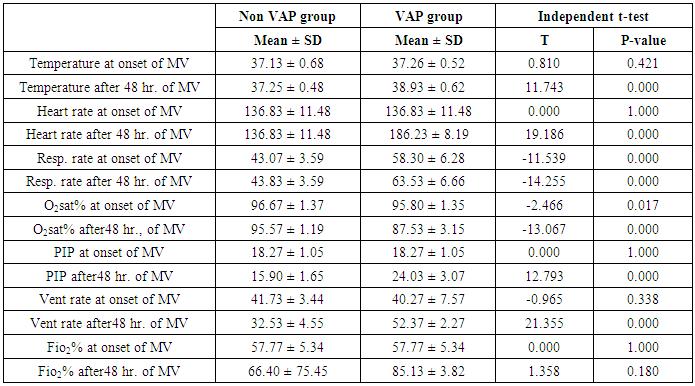
- This study included sixty mechanically ventilated full term infants divided into two groups: thirty mechanically ventilated patients with VAP and thirty mechanically ventilated infants without VAP. There was no statistically significant difference between the two studied groups as regarding age, sex, anthropometric measurements and type of delivery (Table 2).
|
|
|
|
|
|
|
|
|
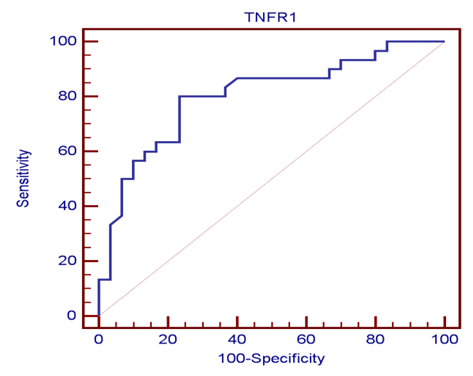 | Figure (1). ROC curve demonstrates TNFR1 as an early marker for diagnosis of neonatal VAP |
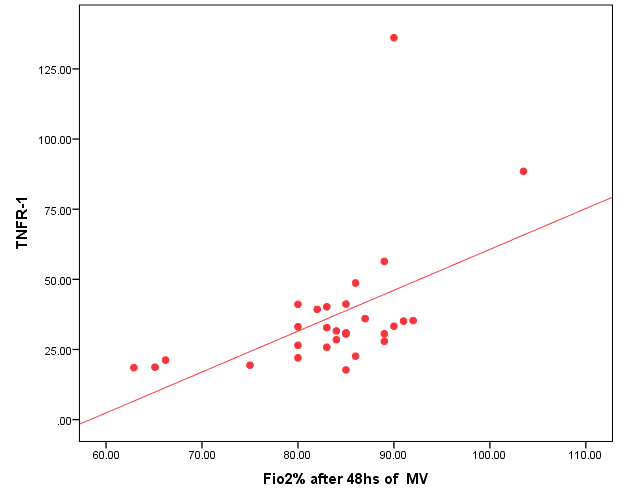 | Figure (2). Positive correlation between TNFR1and FIO2 after 48 hr. of MV |
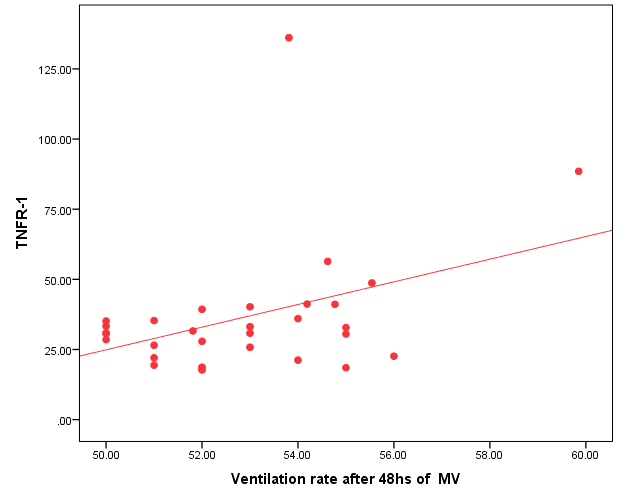 | Figure (3). Positive correlation between TNFR1 and ventilation rate after 48 hr. of MV |
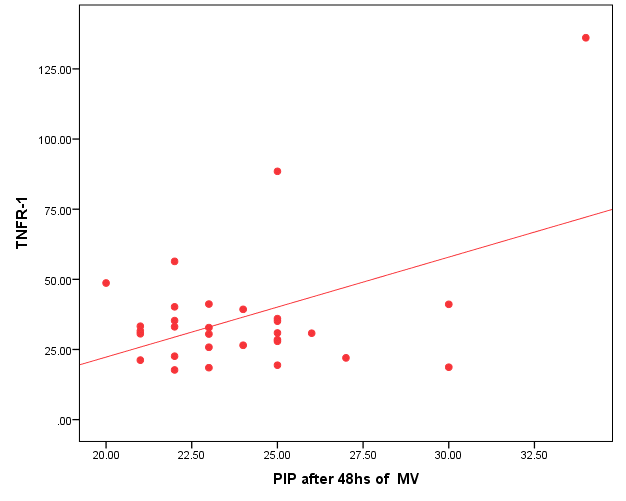 | Figure (4). Positive correlation between TNFR1and PIP after 48 hr. of MV |
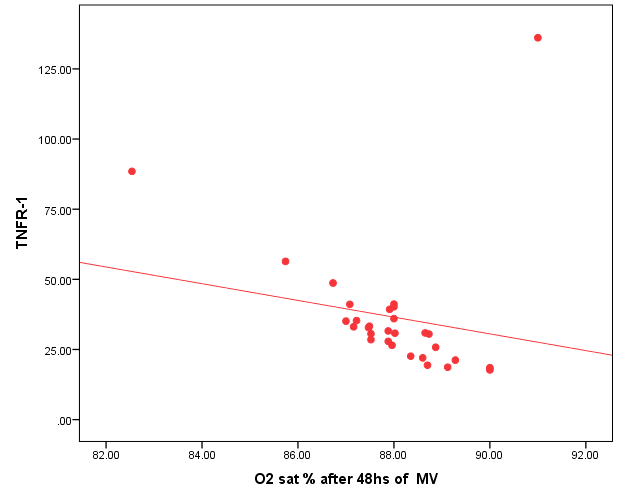 | Figure (5). Negative correlation between TNFR1 and O2 saturation after 48 hr. of MV |
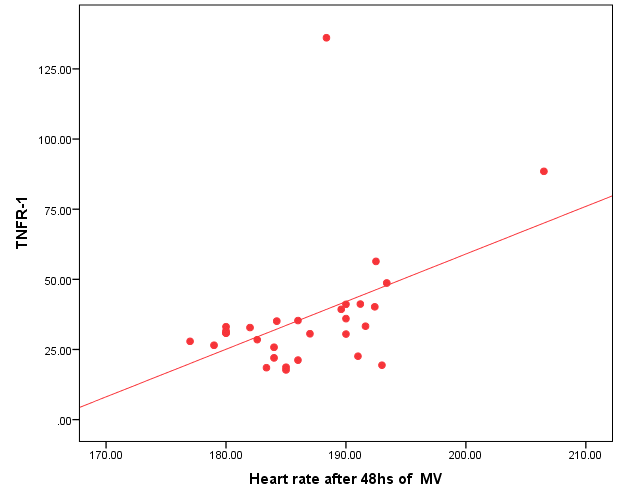 | Figure (6). Positive correlation between TNFR1and heart rate after 48 hr. of MV |
10. Discussion
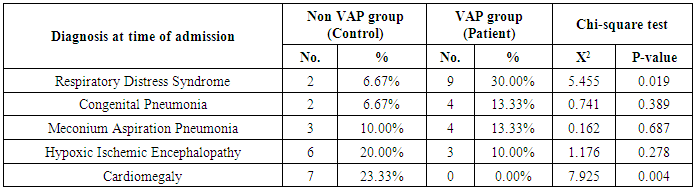
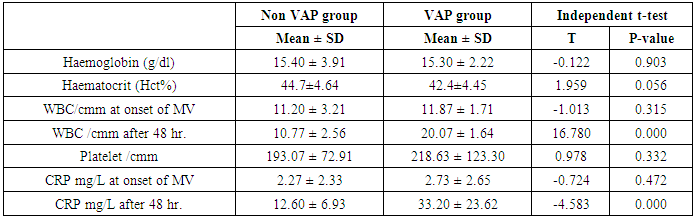
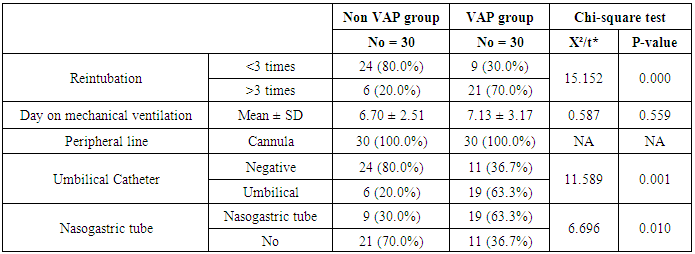
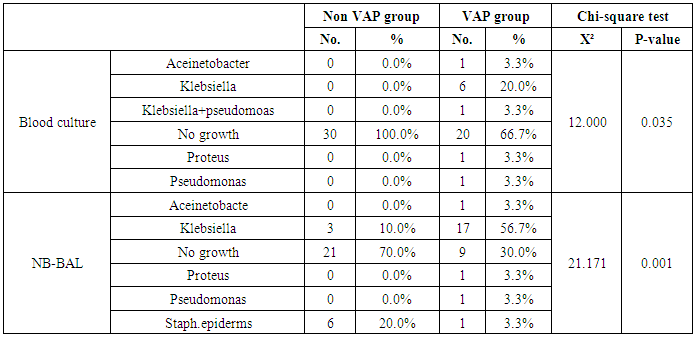
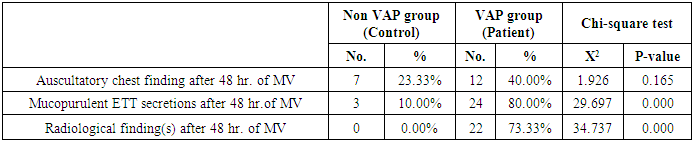
- Ventilator associated pneumonia is a major cause of morbidity and mortality in hospitalized patients. The risk is especially high in the NICU. Neonates have unique characteristics predisposing them to nosocomial infections. These patients’ immature immune systems place them at increased risk for infection. Skin and mucous membranes are more permeable and are less effective barriers to infection. Abnormal granulocyte migration and bacterial digestion in these patients have been demonstrated. Additionally, decreased activity of complement, particularly complement opsonization, occurs in newborns [11].While the diagnosis of VAP is a challenge [12, 13], the most important aspect to improve outcome is early recognition and the initiation of the appropriate empirical treatment. Too late initiation or inappropriate antibiotic therapy is associated with adverse outcomes.Diagnosis and prediction of VAP has been widely studied in order to find a single biomarker that can achieve this goal [14, 15]. To our knowledge, this is the first study that aimed to evaluate TNFR1 as an early biomarker for diagnosis of neonatal VAP.This study included sixty mechanically ventilated full term infants divided into two groups: thirty mechanically ventilated patients with VAP and thirty mechanically ventilated infants without VAP. All patients and controls were subjected to chest X ray, NB-BAL for (cytologic and bacteriologic examination and assay of TNFR1 after 48 hours of mechanical ventilation), Blood sample for Blood culture, CBC and CRP determination. Monitoring and recording of vital signs and mechanical ventilation settings were done for all participants.All participants were of gestational age 37-42 weeks and of normal body weight. There was no significant difference in neonates with and without VAP regarding sex, age, and modes of delivery. We couldn't elicit any significant differences between VAP and non-VAP patients as regards birth weight. Similar to our results, Pessoa-Silva [16], did not find inverse relationship between birth weight and frequency of nosocomial pneumonia. The rate of nosocomial pneumonia was actually higher in neonates with birth weights of >1,500 g than in babies with birth weights of ≤1,500 g. In contrast to our study Tripathi et al, Afify et al. and Lee et al. [4, 17, 18], found that low birth weight, very low birth weight (< 1500 grams) and prematurity (gestational age < 37 week) are important risk factors of developing VAP. Foglia et al [11] Stated that hypogammaglobulinemia occurs in premature newborns may increase the risk of VAP in premature infants as maternal immunoglobulin G (IgG) is transported to the foetus in the second and last trimesters of pregnancy, and foetal IgG levels reach maternal levels by term. In the present study patients with VAP showed higher duration on mechanical ventilation. Similar to our results Awasthi et al [3], reported increased risk of VAP with >4 days of MV. Apisarnthanarak et al [19] stated that prolonged duration of ventilation increases the risk of infection due to the exposure to humidifiers, nebulizers and ventilator circuits that are proven to be important sources and media for microorganisms. In the current clinical practice there was increase in the number of reintubation > 3 times in the VAP group (70%) compared to 20% in non VAP group. So, re-intubation in VAP group was considered one of risk factors for development of VAP.These results were in agreement with (Mir et al, Tripathi et al, Yuam et al, and Livigston) [20, 4, 21, 22] who found increased number of re-intubation in VAP group. The increased incidence of VAP following re-intubation was probably related to enhanced risk of aspiration of colonized oropharyngeal contents during each episode of intubation [23]. In contrast to our results, the study done by Deng et al. [24] found that re-intubation for more than three times was not a risk factor for the development of VAP.In our study, there was no significant difference between the two studied groups regarding the insertion of nasogastric tubes as a risk factor for development of VAP. In contrast to our results, the study done by Badr et al. [25] found that (53%) in VAP group and (25%) in non-VAP group exposed to NGT with (p=0.03). also Mir et al. [20] found that insertion of NGT is a very important risk factor for development of VAP (p=0.01). This is explained by fact that the nasogastric tubes increase stomach colonization with gram -ve microorganisms and consequently increase the rate of nosocomial pneumonia [26]. The current study showed increased percentage of umbilical catheter insertion (63.3%) in VAP group compared to (16.7%) in non VAP with (p=0.001). Similar results were reported by Badr et al [25].In this study, chest radiographs were diagnostic in all cases clinically diagnosed as VAP in which (73.3%) of VAP group after 48hrs of MV were presented with a new chest findings, compared to (25.4%) of non VAP group (p=0.001), which was in agreement with El-ward et al. [27] and Badr et al. [25].In the current clinical practice there were increased ventilator settings (PIP and VR) and decrease in O2 saturation % after 48 hr. of MV compared to the onset of ventilation. These results were in agreement with Garland [28] and Cernada et al. [29] who found that worsening of gas exchange (e.g., oxygen desaturations, increased oxygen requirements, increased ventilatory demand) were clinical criteria of VAP.In the present study, there were significant increase in heart rate, respiratory rate and temperature after 48hrs of MV in VAP group. These results were in agreement with Cernada et al. [5] and Tripathi et al. [4] who found bradycardia (<100 beats/min) or tachycardia (>170 beats/min), temperature instability and worsening of the respiratory distress with no other recognized cause are of the diagnostic criteria for VAP in infants younger than 1 year.In this study, there were significant differences between VAP and non VAP groups regarding total leucocytic count and CRP titre (p= 0.001). These results were in agreement with Afjeh et al. [30] and Povoa et al. [31] who reported significant leukocytosis and increased CRP much more in the neonates developed VAP.In this study, (33.2%) of obtained blood cultures were positive in VAP diagnosed group. This is in agreement with Fallahi et al. [32] who reported that bacteremia or blood stream infection before the onset of VAP is regarded as independent risk factor for VAP.In the current study, the predominant microorganism in blood culture was Klebsiella (20.0%). This is in agreement with Berthelot et al. [33] and Badr et al. [25]. While the study done by Yuan et al. [34] found that the most common pathogens isolated in the neonates with pneumonia are Pseudomonas aeruginosa and Staphylococcus aureus, also Khattab et al. [35] found that the most common pathogens isolated in the neonates with pneumonia are Staphylococcus aureus (15%), Klebsiella spp.(8.5%) Candida spp. (6.5%), Pseudomonas spp. (4.2%), and Escherichia coli (4.2%). While Afich et al. [36] found that blood cultures were negative in all infants diagnosed with VAP (100%).The results of NB-BAL cultures in our study revealed that (70%) were positive with Klebsiella organism predominating the positive culture (34.3%), while Staphylococcus aureus and pseudomonas account were (3.3%) for each one. This agree with Khattab et al. [35] who found that the results of nonbronchoscopic bronchoalveolar lavage cultures revealed the presence of Klebsiella spp. (34%), Pseudomonas spp. (25.5%), S. aureus (17%), E. coli (17%), and Candida spp. (6.4%). K. pneumoniae was the most commonly isolated pathogen in nonbronchoscopic bronchoalveolar lavage. Koksal et al. [37] mentioned that Acinobacter was the most predominating causative agent whereas Petdachai [38] reported that Pseudomonas was the most common organism isolated.Tumor necrosis factor receptor- 1 is a member of the death domain-containing receptor family [39, 40]. It initiates cell activation, differentiation and survival signaling, mainly through the activation of nuclear factor κB and the induction of genes associated with cell survival including Bcl-XL and c-FLIP [41].In our study, levels of TNFR1were significantly higher in bronchoalveolar lavage in VAP group compared to non VAP with the mean TNFR1 was (36.68 ± 23.27) and (26.98 ± 7.33) for VAP group and non VAP respectively (p=0.036) The cut-off point, sensitivity and specificity of TNFR1 as an early biomarker for diagnosis of neonatal VAP is >29.9 ug/ml with 63.3% sensitivity and 65.5% specificity.Martin-Loeches et al. [42] found that the combination of TNFR1 and Granulocyte colony stimulating factor (GCSF) achieved a very good sensitivity and specificity (sensitivity: 96%, specificity: 87%) at the day of diagnosis of VAP on study done on adult patient developing VAP.In the present study there was a positive correlation between TNFR1 in BAL and mechanical ventilator setting (PIP, VR and Fio2) and clinical sings (Heart rate) and negative correlation between TNFR1 and O2 saturation after 48 hr. of MV in VAP group. This means, the more increase of TNFR1 in NB-BAL in VAP, the more the severity of the condition. This may be explained by the more inflammatory process associated with increased severity of VAP.
11. Conclusions
- Our study demonstrated that TNFR1 in BAL is a sensitive marker for diagnosis of neonatal VAP with 80% sensitivity and 76.7 % specificity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML