-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(4): 103-109
doi:10.5923/j.cmd.20160604.03

Correlation between Antiphospholipid Antibodies and the Intima Media Thickness in Vascular access Thrombosis in Egyptian Patients with Systemic Lupus Erythematosus on Regular Hemodialysis
Osama Mohamad Ahmad1, Ayman Abdel Aziz2, Ahmed Salama Al Adl1, Mahmoud Saeed1, Hamdi S. Nasser3, Hesham H. Amin4
1Internal Medicine Department, Faculty of Medicine, Al-Azhar University, Damietta, Egypt
2Internal Medicine Department, Faculty of Medicine, Al-Azhar University, Assuit, Egypt
3Rheumatology, Physical Medicine and Rehabilitation Department, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
4Clinical Pathology Department, Faculty of Medicine, Al-Azhar University, Assuit, Egypt
Correspondence to: Ahmed Salama Al Adl, Internal Medicine Department, Faculty of Medicine, Al-Azhar University, Damietta, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Anticardiolipin antibody is associated with increased risk of vascular access thrombosis in hemodialysis (HD) patients. Thus the thrombosis risk factors should be evaluated extensively and regularly and treated aggressively in every patient with systemic lupus erythematosus on regular hemodialysis. Aim of the work: Assessment the occurrence of vascular access thrombosis in SLE patients on regular hemodialysis, and its relation to SLE disease activity and correlation of antiphospholipid antibodies to occurrence of vascular access thrombosis. Patients and methods: This cross-sectional study included 50 Egyptian patients with SLE on HD, who had either an arterio-venous (AV) graft or naive AV fistula as vascular access, and were not on anticoagulation treatment, were recruited from dialysis unite of Al Azhar university Hospital new Damietta, hemodialysis unite of Al Azhar university Hospital Assuit, Kafr saad general Hospital hemodialysis unite, and other specialized Hemodialysis unites in Damietta; during the period from November 2013 to October 2014; patients were divided in to two groups according to presence or absence of vascular thrombosis; Group A: Included thirty-four patients with SLE on regular hemodialysis with previous thrombosis (Thrombotic Lupus). Group B: Included sixteen patients with SLE on regular hemodialysis with no previous thrombosis and/or fetal loss (Non-Thrombotic Lupus). Also there was Control group which included 20 patients with end stage renal disease (ESRD) on regular hemodialysis without SLE. All the studied subjects underwent full history taking. detailed clinical examination, measurement of anti-ds DNA, ANA, anticardiolipin IgG & IgM and lupus anticoagulant in addition to CBC, PT, PTT, kidney, liver function tests and duplex on A-V fistula with measurement of intima media thickness. Univariate. Results: The results showed that 68% of SLE patients had previous thrombosis, and 32% of SLE patients had no thrombosis. there were a highly statistically significant difference between the three groups as regard ACL IgG & IgM, lupus anticoagulant, ANA, Anti-ds DNA and duplex on A-V fistula (P < 0.01) as it was highest in group A and lowest value detected in group B. there were a highly statistically significant positive correlations between ANA and PTT and between ACL IgG and ACL IgM and lupus anticoagulant (P < 0.01). Conclusions: The levels of Anti cardiolipin IgG, IgM, lupus anticoagulant were higher in SLE patients on regular hemodialysis with vascular access thrombosis with high correlation to intima media thickness of the vascular access. And the occurrence of thrombosis is highly related to SLE disease activity.
Keywords: Systemic lupus erythematous, Vascular access thrombosis
Cite this paper: Osama Mohamad Ahmad, Ayman Abdel Aziz, Ahmed Salama Al Adl, Mahmoud Saeed, Hamdi S. Nasser, Hesham H. Amin, Correlation between Antiphospholipid Antibodies and the Intima Media Thickness in Vascular access Thrombosis in Egyptian Patients with Systemic Lupus Erythematosus on Regular Hemodialysis, Clinical Medicine and Diagnostics, Vol. 6 No. 4, 2016, pp. 103-109. doi: 10.5923/j.cmd.20160604.03.
Article Outline
1. Introduction
- Systemic lupus erythematosus (SLE) is an autoimmune disease with diverse clinical manifestations that primarily affects young women. Women are affected nine times more frequently than men. Vascular access failure is one of the leading causes of morbidity, mortality and hospitalizations in ESRD [1]. It is not clear that SLE patients are at a higher risk for vascular access complications though the one study on the subject suggests it. That retrospective study compared rates of vascular access thrombosis in 36 SLE patients and 36 non-SLE controls, matched for age, sex, race and vascular access and found a 66.6% rate in SLE compared with 38.9% in non-SLE patients. This study did not identify the factors predictive of vascular access thrombosis in lupus patients [2]. While vascular access complications are multifactorial and related to both genetic and acquired factors, one possible mechanism of increased vascular access thrombosis in SLE may be the presence of antiphospholipid antibodies (aPL Abs). aPL Abs are a heterogeneous group of Abs directed against phospholipids (such as cardiolipin) or phospholipid-binding proteins (such as β2-glycoprotein I or annexin A5). Over 30% of SLE patients develop persistently positive aPL Abs during the course of their disease. Persistently positive aPL Abs are associated with an increased risk of arterial and/or venous thrombosis, as well as complications during pregnancy. While this condition, known as antiphospholipid syndrome, may occur in individuals without SLE, it occurs with a higher frequency in SLE patients. Although aPL Abs may be present for a long time before an acute thrombotic event, it is widely accepted that they play an active role in the pathogenesis of antiphospholipid syndrome [3, 4]. However, not all aPL Abs are pathogenic, and some may merely reflect endothelial damage in inflammatory states such as SLE and ESRD [5, 6]. The exact mechanisms of thrombosis in antiphospholipid syndrome are not well understood.
2. Aim of the Work
- Assessment the occurrence of vascular access thrombosis in SLE patients on regular hemodialysis and its relation to SLE disease activity. And correlation of antiphospholipid antibodies to occurrence of vascular access thrombosis.
3. Patients and Methods
3.1. Study Design
- The present cross-sectional study included Egyptian patients with SLE (N = 50) with ESRD who fulfilled 1997 revised criteria of the American College of Rheumatology (ACR) for SLE, All patients and control undergoing HD therapy for more than six months at dialysis unite of Al Azhar university Hospital new Damietta, hemodialysis unite of Al Azhar university Hospital assuit, Kafr saad general Hospital hemodialysis unite, and other specialized Hemodialysis unites in Damietta during the period from November 2013 to October 2014; patients were divided in to two groups according to presence or absence of vascular thrombosis Group A: Included thirty-four patients all were females with SLE on regular hemodialysis with previous thrombosis. Group B: Included sixteen patients all were females with SLE on regular hemodialysis with no previous thrombosis and/or fetal loss. Also there was Control group which included 20 females’ patients with end stage renal disease (ESRD) on regular hemodialysis without SLE.
3.2. Ethical Aspects
- The informed consent was obtained from all participants. The research protocol did not interfere with any medical recommendations or prescriptions.
3.3. Exclusion Criteria
- Patients receiving oral anticoagulation therapy or oral contraceptives, chronic hepatic disease, malignant diseases, vasculitis, acute infections, history of renal transplantation, HIV positive and pregnant women were automatically excluded from the study.
3.4. Inclusion Criteria
- We allocated HD patients with arteriovenous fistula (AVF) into two groups according to the occurrence of previous episode of vascular access thrombosis. The case group consisted of 50 patients whose functioning dialysis access had, at least, one previous episode of thrombotic occlusion, which was defined by the absence of blood flow and the impossibility to use the access for dialysis(by history examination of AVF, and Douplex).
3.5. Study Protocol
- All patients required regular HD sessions for 4 h, three times a week. Blood flow was 300–350 mL/min with a dialysate flow at a constant rate of 500 mL/min. Patients were dialyzed either with low-flux polysulphone membranes and high-flux membranes with bicarbonate-buffered dialysate. All patients received regular doses of standard heparin (100 to 150 UI/kg) during hemodialysis session. A detailed history, clinical variables (age, gender, BMI, pre-dialysis blood pressure levels, etiology of ESRD, presence of diabetes or not, type of vascular access, time on hemodialysis, interdialytic weight gain, and main medications in use) and dialysis parameters (urea reduction ratio and normal protein catabolism rate) of each included patient were recorded in a computer specific data bank. After informed consent, all patients were submitted to: Anti-ds DNA, Anti-cardiolipin IgG & IgM, Lupus anticoagulant, CBC, liver function testes, PT, PTT, S. creatinine, Blood Urea Nitrogen, Serum uric acid, ESR.
3.6. Anti-ds DNA, Anti-cardiolipin IgG & IgM and Lupus Anticoagulant
- Anti-ds DNA antibodies were determined by enzyme-linked immunoassay technology (ELISA) supplied by Calbiotech, Inc (CA) serum values ≤26 IU/ml were considered negative and values >26 IU/ml were considered positive.ANA: Anti-nuclear antibodies were determined by enzyme-linked immunoassay technology (ELISA) supplied by Abcam 178610. Serum values ≤1/60 were considered negative and values >1/60 were considered positive. Anti-cardiolipin IgG & IgM antibodies were determined by ELISA using anti-cardiolipin IgG & IgM (ABIN770596) kits. Specimen Collection, Storage and Handling: 3 ml of blood was collected in plain vacutainers for analysis of Anti-cardiolipin IgG & IgM antibodies. Serum samples were stored at -20°C until the time of assay. Serum values up to 23 (µ/ml) consider normal for Anti-cardiolipin IgG, and up to 11 (µ/ml) consider normal for Anti-cardiolipin IgM Lupus anticoagulant: It is an immunoglobulin that binds to phoshpolipid associated with cell membrane. It is a misnomer, as it is actually a prothrombotic agent in vivo but in vitro, it is anticoagulant. These antibodies cause an increase in aPTT. The initial workup of a prolonged PTT was a mixing test whereby the patient’s plasma was mixed with normal pooled plasma and the clotting re-assessed. Prolonged clotting tests was present denoting the presence of lupus anticoagulant and the diagnosis confirmed by the dilute Russell's viper venom time which was done by mixing pooled normal plasma with diluted PL at 37°C then dieted RVV and calcium chloride were added and the clotting time was measured. The test was repeated using patient plasma by using DRVVT, LAC 115 kits. Normal range of lupus anticoagulant = 30-42 sec.Duplex on Arterio -Venous fistula with measurement of intima- media thickness: was done by using Toshiba Real Time duplex Ultrasound: Normal intima media thickness = 57± 42 µm.All data were collected and tabulated for further analysis.
3.7. Statistical Methods
- Data were analyzed using SPSS 18 (SPSS Inc., USA); the following tests were done: Comparison between two independent mean groups for parametric data using Student’s t test. Comparison between two independent groups for nonparametric data using Mann-Whitney test. Chi-square test to study the association between each 2 variables or comparison between 2 independent groups as regards the categorized data. Correlations were done using Pearson correlation co-efficient. Analysis of variance between groups by using one-way analysis of variance (ANOVA) test; the post-hoc least significant difference (LSD) was calculated for comparison between two groups (P < 0.05 = Significant and P < 0.01 = highly significance).
4. Results
- The results showed 68% of SLE patients had history of previous thrombosis, and 32% of SLE patients had no thrombosis. There was a highly statically significant difference between the three groups as regard previous abortion and taking steroid therapy being highest in group A (P < 0.05).There was a highly statistically significant difference between group A & B as regard musculoskeletal (being highest in group B) and cardio-respiratory manifestations (being highest in group A) (P < 0.01).There were a highly statistically significant difference between studied groups as regard Hb, PT, PTT, ESR, ACL IgG & IgM, lupus anticoagulant, ANA, Anti-ds DNA and duplex on A-V fistula (P < 0.01) as it was highest in group A and lowest value detected in group B but there were no statistically significant difference between studied groups as regard WBCs, PLT, ALT, AST, S.Cr, BUN (P > 0.05).There were a highly statistically significant positive correlations between ANA and PTT and between ACL IgG and ACL IgM and lupus anticoagulant (P < 0.01) but there were no statistically significant correlations between other items (P > 0.05). There was significant negative correlation between intima media thickness measured by duplex on Arteio-Venous fistula and platelet count (P < 0.01).
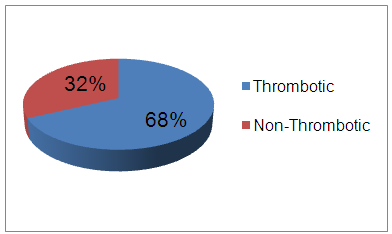 | Figure (1). SLE patients with or without thrombotic events |
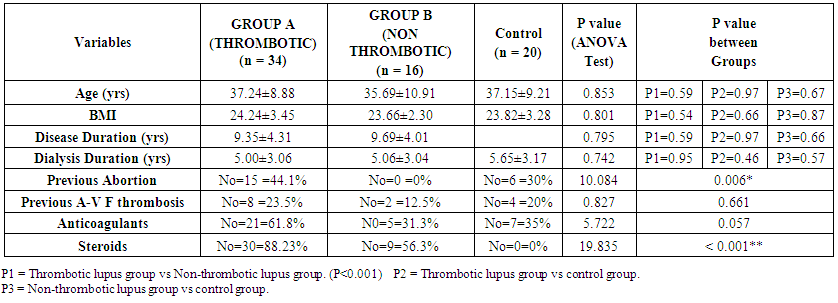 | Table (1). Statistical comparison between studied groups as regard to different variables |
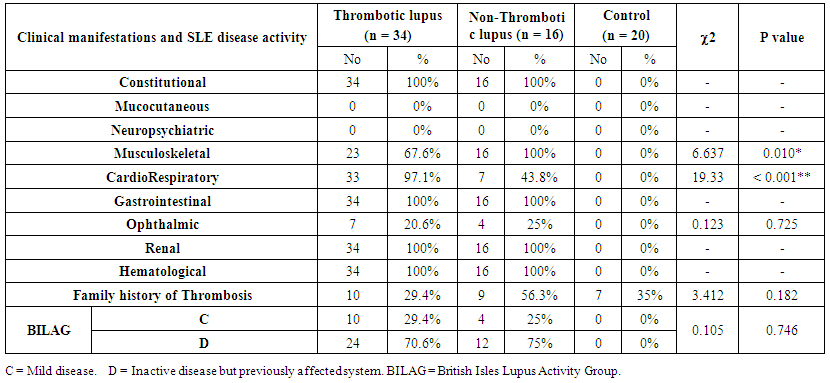 | Table (2). Statistical comparison between studied groups as regard to clinical manifestations and SLE disease activity (BILAG) |
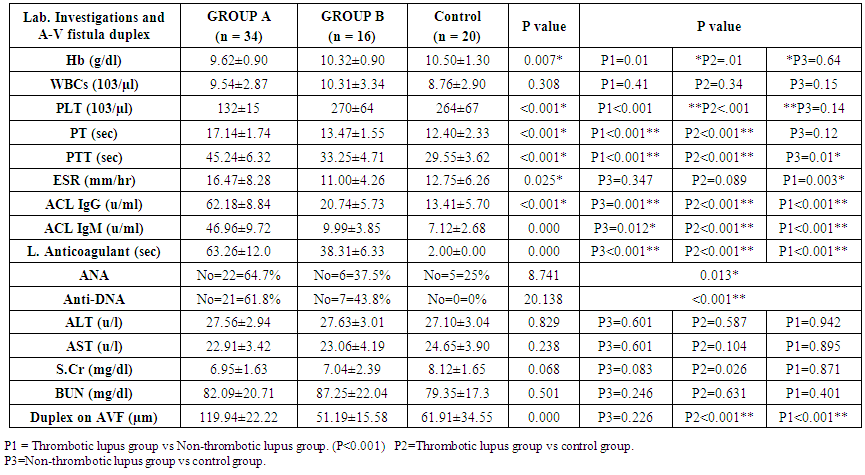 | Table (3). Comparative study between SLE patients with & without thrombosis and control groups as regard lab. Investigations and A-V fistula duplex by using ANOVA test |
5. Discussion
- Systemic lupus erythematosus (SLE) is heterogeneous multi-system disease characterized by auto-antibodies production and impaired immune complex clearance [7].There is a common perception among rheumatologists that SLE becomes clinically inactive after ESRD develops, due to immune system alterations associated with uremia and ESRD. Despite this common perception, robust experimental and epidemiologic data are lacking.ESRD is associated with complex alterations of innate and adaptive immunity contributing to accelerated atherosclerosis and increased susceptibility to infection [8]. On the one hand, ESRD is associated with increased macrophage activation, increased oxidative stress, and up-regulation of inflammatory cytokines [9]. On the other hand, there is decreased monocyte and neutrophil function and immune deficiency caused by depletion of dendritic cells, B cells and T cells [8].Our cross-sectional study was conducted on 50 females SLE patients divided according to presence or absence of thrombosis into 2 groups; group A which included 34 patients with end stage renal disease and known as thrombotic group, their ages (mean 35.48, SD ± 9.17) years and duration of SLE (mean 9.43, SD ± 4.35) years, also there were previous thrombosis and/or abortion in all patients and 58.8% of them taking steroids (mean 10.2, SD ± 3.4) mg. and group B which included 16 patients with end stage renal disease and known as non-thrombotic group, their ages (mean 35.71, SD ± 13.98) years and duration of SLE (mean 10.57, SD ± 5.38) years, also there were no previous thrombosis and/or abortion in all patients and 56.3% of them taking steroids (mean 8.42, SD ± 4.2) mg. and control group which included 20 females patients with end stage renal disease (ESRD) on regular hemodialysis without SLE.The serum level of Anti-cardiolipin IgG was statistically significant increase in patients of group A (mean 62.03, SD ± 9.69) U/ml than in patients of group B (mean 20.17, SD ± 3.35) U/ml. and serum level of Anti-cardiolipin IgM was statistically increased in patients of group A (mean 46.90, SD ± 11.18) U/ml than in patients of group B (mean 9.09, SD ± 2.12) U/ml and we found that 68% of SLE patients had previous thrombosis, and 32% of SLE patients had no thrombosis these in agreement with Shovman et al. 2007 [10] who made a study on 15 SLE patients and 53.3% was aCL+ve with multiple venous and arterial thrombosis. Also in agreement with Silverstein et al. 2009 [11] who performed a study on 12 SLE patients with 41.6% of them had aCL+ve and 40% of patients with aCL+ve had cerebral venous thrombosis. Petri et al. 2012 [12] who followed 70 SLE female patients with end stage renal disease on regular hemodialysis, anti-cardiolipin IgG and IgM were elevated in 70% of them and their values were (mean 83.5, SD ± 7.8) U/ml and (mean 62.1, SD ± 5.3) U/ml respectively. Also there was a highly statistically significant difference between group A & B as regard lupus anticoagulant in patients of group A (mean 64.14, SD ± 13.69) sec, while in patients of group B (mean 37.71, SD ± 4.99) sec and this coming in accordance with Herbert et al. 2014 [13] who performed a study on 14 SLE patients and 28.5% of them had positive values of lupus anticoagulant (mean 87.9, SD ± 9.2) sec and had more thrombotic insults so lupus anticoagulant were one of the most common acquired predisposing causes of thrombosis.In agreement with Marti et al. 2010 [14] who found about 30% of SLE patients have anti-cardiolipin antibodies and about 25% have lupus anticoagulant.We also found a highly statistically significant difference between group A & B as regard PTT which is prolonged in patients of group A (mean 46.19, SD ± 6.36) sec, versus in patients of group B (mean 32.43, SD ± 3.21) sec and this came accordance with Herbert et al. 2014 [13] who performed a study on 7 patients with aCL+ve and 7 aCL –ve patients and found that there was prolongation in PTT (mean 55.19, SD ± 8.31) in all patients with aCL +ve and normal value of PTT in aCL –ve patients.In agreement with Del Ross et al. 2013 [15] who performed a study on 20 SLE patients on hemodialysis with thrombotic events and found that 20% of them had aCL+ve with prolonged PT & PTT. This meant a great association between anti-phospholipid syndrome and prolonged PT & PTT.There was significant negative correlation between lupus anticoagulant and PTT and this agreed with Herbert et al. 2014 [13].In this study there was a highly statistically significant difference between group A & B as regard PT which is prolonged in patients of group A (mean 17.48, SD ± 1.57) sec, versus in patients of group B (mean 13.29, SD ± 1.25) sec and this came in accordance with Herbert et al. 2014 (13) who performed a study on 7 patients with aCL+ve and 7 aCL –ve patients and found that there was prolongation in PT (mean 20.54, SD ± 2.87) in all patients with aCL +ve and normal PT value in aCL –ve patients.We found that intima media thickness by duplex on Arterio-Venous fistula was statistically significant increase in patients of group A (mean 118.54, SD ± 21.13) µm, than in patients of group B (mean 50.77, SD ± 14.71) µm in agreement with Holers et al. 2011 [16] who performed a study on SLE females patients on hemodialysis with positive and negative anti-cardiolipin antibodies with measurement of intimal media thickness of A-V fistula by duplex and found that there was increased in patients with aCL +ve (mean 102.21, SD ± 15.7).In our study there was a highly statistically significant difference between group A & B as regard to anti-ds DNA as 61.8% of group A patients had positive anti-ds DNA versus 43.8% in group B and this came in accordance with Leong et al. 2012 [17] who performed a study on 30 patients with positive and negative aCL antibodies and found that 66.6% patients with aCL +ve had positive anti-ds DNA and 33.3% patients with aCL –ve, 60% of them had –ve anti-ds DNA and 40% of them had +ve anti-ds DNA.We found thrombocytopenia in patients of group A (mean 130, SD ± 15), while in patients of group B (mean 270, SD ± 64) in agreement with Bazzan et al. 2013 [18] who performed a study on 15 thrombotic and 10 non-thrombotic SLE patients on hemodialysis and found that 75% of thrombotic had thrombocytopenia (mean 90, SD ± 20.56) while non-thrombotic patients did not had thrombocytopenia (mean 300, SD ± 50.98).There was significant negative correlation between intima media thickness measured by duplex on Arteio-Venous fistula and platelet count in agreement with Lucia et al. 2010 [19] who made a study on SLE patients on hemodialysis with increased intima media thickness of A-V fistula and found that there was significant thrombocytopenia (mean 123.45, SD ± 17.36) in these patients.We found that ESR was elevated in patients of group A (mean 16.47, SD ± 8.28), while in patients of group B (mean 11.00, SD ± 4.26).We also found that 29.4% of group A and 25% of group B had BILAG index C which indicated mild disease activity and 70.6% of group A and 75% of group B had BILAG index D which indicated inactive disease but previously affected system and this came in accordance with Yee et al., 2008 [20] who performed study on 25 SLE patients with ESRD on hemodialysis with and without thrombosis and found that 32% of thrombotic and 24% of non-thrombotic had BILAG index C and 68% of thrombotic and 76% of non-thrombotic had BILAG index D. Also there was highly statistically significant difference between group A & B as regard taking steroid therapy as 88.23% of group A and 56.3% of group B were taking steroids and this indicate high activity in group A than group B.ALT and AST had no statistically significance between group A and B with (mean 27.62, SD ± 2.20) and (mean 27.11, SD ± 3.41), (mean 22.62, SD ± 3.19), (mean 23.56, SD ± 4.00) respectively in agreement with Michael et al. 2012 [21] who found that liver enzymes not usually elevated in SLE patients. In this study there was no statistically significant difference as regard SLE disease duration between patients of group A (mean 9.43, SD ± 4.35) and group B (mean 9.00, SD ± 2.69) in agreement with Ibrahim Al-Hamood, 2012 [22] who made his study on two groups of SLE patients, aCL +ve and aCL –ve groups with SLE duration (mean 6.5, SD ± 3.64) and (mean 6.1, SD ± 3.3) respectively and there was no relation between SLE duration and occurrence of vascular thrombosis.Also as regard hemodialysis duration there was no obvious significance between patients of group A (mean 4.71, SD ± 2.95) and group B (mean 4.22, SD ± 2.64) which agreed with Brunet et al. 2008 [23] who performed their study on 97 SLE patients on hemodialysis dividing them into thrombotic and non-thrombotic with hemodialysis duration (mean 5.6, SD ± 2.4) and (mean 5.31, SD ± 2.7) respectively and found that there were no relation between duration of hemodialysis and thrombosis. Also there was apparently difference but no statistically significant difference between group A & B as regard family history of thrombosis as 29.4% of group A patients had family history of thrombosis versus 56.3% in group B.The clinical manifestations were highly statistically significant difference between groups as regard musculo-skeletal and cardio-respiratory manifestations, but there were no statistically significant difference between groups as regard other manifestations and this coming in accordance with Petri et al. 2012 [12] who performed a study on 40 SLE patients on hemodialysis and found that musclo-skeletal and cardio-respiratory manifestations were the most prominent in 60% of them.
6. Conclusions
- The levels of Anti cardiolipin IgG, IgM, lupus anticoagulant were higher in SLE patients on regular hemodialysis with vascular access thrombosis with high correlation to intima media thickness of the vascular access. And the occurrence of thrombosis is highly related to SLE disease activity.
Abbreviations

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML