-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(3): 62-69
doi:10.5923/j.cmd.20160603.04

Study of Circulating Endothelial Progenitor Cells and Endothelial Dysfunction in Type 2 Diabetes Mellitus Patients
Rabie Fathy Abbas1, Mamdouh Attia Mohammed2, Mohmmed Sayed Ahmed Attia3
1Internal Medicine Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt
2Clinical Pathology Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt
3Radiodiagnosis Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt
Correspondence to: Rabie Fathy Abbas, Internal Medicine Department, Faculty of Medicine (Boys), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Endothelial dysfunction predicts cardiovascular events and represents an underlying event for vascular abnormalities observed in type 2 diabetes mellitus (T2DM) patients. Vascular endothelial dysfunction is an early marker of atherosclerosis seen in T2DM. It is suggested that-endothelial dysfunction and injury in the vascular wall are repaired by bone marrow-derived endothelial progenitor cells (BM-derived EPCs). Endothelial progenitor cells (EPCs) are mobilized from BM into the peripheral blood in response to tissue ischemia or injury. Aim of the work:This study was designed to assess the relationship of circulating EPCs and endothelial dysfunction in T2DM. Patients: After departmental ethics committee approval and participants consent were obtained, 60 diabetic patients having variable duration of T2DM (group-I) and 30 healthy persons (group-II) with matched age and sex were enrolled in the study. Methods: All participants were subjected to: Detailed history taking, full clinical examination and laboratory studies including; fasting and 2-hour-post-prandial plasma glucose (FPG and PPPG), glycated hemoglobin A1c (HbA1c), lipids profiles, quantification of circulating (EPCs) with flow-cytometry, assessment of vascular endothelial function with flow-mediated dilatation percentage (FMD%) of the brachial artery with a high-resolution ultrasound system. All studied parameters were collected and analyzed statistically using SPSS version 12.Results: Group I (diabetics): 60 T2DM patients with variable duration of DM ranged between 1 and 10 years (33 females and 27 males); their age ranged between 35 and 66 years old. Group II (controls): 30 healthy persons (15 females, 15 males); their age ranged between 30 and 65 years old. The obtained results showed that, Significant increase of systolic, diastolic blood pressures and lipid profiles in diabetics compared with controls. However, significant decrease in EPCs (CD34+, CD133+, and CD34 + CD133+) and FMD% in diabetics compared with controls (P < 0.05). In diabetics, there were inverse correlations between CD34+ counts and advancing age of patients (r = - 0.400; P < 0.05), total cholesterol (r = - 0.294; P < 0.05), FPG (- 0.578; P < 0.05), 2h-PPPG (- 0.474; P < 0.05), HbA1c (- 0.449; P < 0.05) and duration of DM (r = - 0.381; P < 0.05). CD34+ counts had significant positive correlation with FMD% of diabetics (r = 0.565; P < 0.05), Similarly,CD133+ and CD34+ CD133+ counts had the same correlations. FMD% had significant inverse correlations with age (r = - 0.501; P < 0.05), duration of DM (r = - 0.562; P < 0.05), LDL (r = - 0.309; P < 0.05) and markers of glycemic control [FPG (- 0.582; P < 0.05), PPPG (-0.521; P < 0.05) and HbA1c (- 0.339; P < 0.05)]; all of these in diabetics point to poor glycemic control, advancing age and duration of DM were associated with more impaired endothelial function. Conclusions: Patients with T2DM had close relationship between reducing numbers of circulating EPCs and the degree of endothelial dysfunction independent of traditional cardiovascular risk factors. Quantification of EPCs counts might be considered as a reliable diagnostic modality for early detection of vascular complications in asymptomatic T2DM patients.
Keywords: T2DM, Circulating endothelial progenitor cells (EPCs), Endothelial dysfunction
Cite this paper: Rabie Fathy Abbas, Mamdouh Attia Mohammed, Mohmmed Sayed Ahmed Attia, Study of Circulating Endothelial Progenitor Cells and Endothelial Dysfunction in Type 2 Diabetes Mellitus Patients, Clinical Medicine and Diagnostics, Vol. 6 No. 3, 2016, pp. 62-69. doi: 10.5923/j.cmd.20160603.04.
1. Introduction
- The incidence of T2DM is increasing at an alarming rate, nationally and worldwide [1]. Endothelial dysfunction predicts cardiovascular events [2] and represents an underlying event for vascular abnormalities observed in T2DM patients [3]. The circulating endothelial progenitor cells [EPCs] count has also been proposed as a surrogate marker of vascular dysfunction and is reduced in patients with various cardiovascular risk factors [4]. EPCs are responsible for angiogenesis and maintenance of micro-vascular integrity, the number of EPCs is correlated with oxidative stress [5]. Several lines of evidence indicate that EPCs constitute an important endogenous system that maintains endothelial integrity and vascular homeostasis [6]. Evidence suggested that CD133+CD34+KDR (vascular endothelial growth factor-receptor 2) (VEGF-R2) EPCs were mobilized from bone marrow (BM) into the peripheral blood in response to tissue ischemia or injury [7], these cells migrate to sites of damaged endothelium and differentiate into endothelial cells [ECs] [8], thereby improving blood flow and tissue repair. EPCs contribute to re-endothelialisation and neo-vascularization [6]. EPCs also produce various pro-angiogenic cytokines and growth factors, promoting proliferation and migration of pre-existing ECs, activating angiogenesis and contributing to vascular regeneration and reestablishment of tissue homeostasis [9]. Thus, EPCs may work not only through the activation and support of vasculogenesis but also through the activation and mediation of angiogenesis [10]. DM is associated with EPCs impairment that leads to a wide range of vascular complications, moreover, the endogenous microenvironment in DM having over production of reactive oxygen species (ROS) caused by increased activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase which involved in the initiation and progression of diabetic vascular complications by decreasing the bioavailability of nitric oxide (NO). Blockade of NADPH oxidase restores the functional activity of human CD34+ including its migration and homing capacity into target tissue [11]. Several in vitro studies on EPCs from T2DM patients revealed that oxidized LDL reduced EPC survival, count and function as well as their endothelial nitric oxide synthase (eNOS) activity and NO bioavailability [12]. Micro- and macro-vascular diabetic complications arise from excess damage through well-known biochemical pathways. Interestingly, microangiopathy hits BM microenvironment with features similar to retinopathy, nephropathy and neuropathy. The early onset of BM defect in the natural history of DM was also suggested by a study showed that CD34+ cells started to decline in pre-diabetes and showed first nadir in newly diagnosed T2DM [13]. Endothelial dysfunction can result from and/or contribute to several disease processes, as occur in DM, hypercholesterolemia and hypertension and also due to environmental factors, such as, smoking tobacco products and exposure to air pollution. The hallmark of endothelial dysfunction was impaired NO bioavailability [14]. Many studies stressed on, the strong link between the cardiovascular disease (CVD) complications and DM and described a close association between micro-vascular complications and DM, suggesting that, excess free fatty acids (FFAs), the loss of endothelium-derived NO, insulin resistance (IR), the pro-thrombotic state, endothelial dysfunction, the abnormal release of endothelial vasoactivators, vascular smooth muscle dysfunction, oxidative stress and micro-ribonuclic acids (mi-RS) down-regulation participated in vessel generation, vessel recovery, as well as endothelium balance generated diabetic CVD complications by these major mechanisms of phosphatidyl-inositol 3 kinase, mitogen-activated protein kinase, polyol, hexosamine, generation of advanced glycosylation end products (AGEs) and protein kinase C (PKC) pathways activation [15-16]. Prolonged hyperglycemia and also transient acute hyperglycemia has been proven to impair endothelial function in both macro-and micro-vascular beds in human subjects [17]. Endothelial function in clinical research is usually tested by vascular reactivity studies, measuring the degree of vasodilatation as the changes in diameter induced by specific stimuli in the macro-circulation (e.g. in the brachial arteries) and the microcirculation (coronary, peripheral muscle, subcutaneous and skin microcirculation) [14].
2. Subjects
- Ninety participants were enrolled in this study; Group I (diabetics) includes: 60 patients with T2DM, (33 females and 27 males) having variable duration of DM, either currently on oral hypoglycemic agents or insulin, their age ranged between 35 and 66 years old. Group II (controls) includes: 30 healthy subjects (15 females and 15 males), their age ranged between 30 and 65 years old. All subjects were selected from outpatient clinics of internal medicine department at Sayed Galal Al-Azhar University Hospital. The study was conducted during the period from July 2013 to July 2015, after departmental ethical committee approval and participants consent were obtained. Exclusion criteria: Patients with poorly controlled DM (HbA1c > 11%), or with chronic kidney disease and with heart disease, e.g., dilated cardiomyopathy, recent acute coronary syndrome or coronary revascularization, also, patients with cerebrovascular stroke.
3. Methods
- All participants involved in this study were subjected to; detailed history taking with stress on duration of DM, hypoglycemic drug intake either oral or insulin, full clinical examination including; body mass index (BMI) determination, cardiovascular, respiratory, abdominal and neurological systems examination. FPG, 2h-PPPG and HbA1c. Lipid profile including: Total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein (HDL), Clinical and laboratory evaluations were done to guide in selection of the studied groups and to assess the glycemic control and dyslipidemic states. Quantification of circulating endothelial progenitor cells (EPCs) with immunophenotyping by flowcytometry: Peripheral blood EPCs were analyzed with flowcytometry for the expression of surface antigens CD34+, CD133+ and dual expression of CD34+CD133+ with direct 2-color flowcytometric (FACS caliber BD Biosciences, Franklin Lakes, NJ, USA). Three populations of EPCs; CD34+, CD133+ and CD34+CD133+ were measured with flowcytometry: Fluorescence-activated cell analysis was performed to determine the number of EPCs. Briefly, 100 ul of peripheral blood was mixed with phycoerythrin- conjugated monoclonal antibody against human CD133+ (Sigma, St Louis, USA), followed by a fluorescein isothiocyanate (FITC)-conjugated CD34+ antibody (Beckman Coulter, Fullerten, USA). FITC-labeled IgG1a and PE-labeled IgG2b (monoclonal mouse antibodies) served as the isotypic control for color compensation. Analysis was performed with an automated fluorescence-activated cell counter in which 10000 events were counted the absolute number of cells expressing CD34+, CD133+, and CD34+ CD133+ per 10000 events in the lymphocyte gate was calculated. Assessment of vascular endothelial function through measurement of flow-mediated dilatation percentage (FMD%) using high resolution B-mode Doppler ultrasound (U/S): Endothelial function was assessed with high-resolution B-mode Doppler [ATL-HDI 5000 equipped with a 7.5 MHz linear- array imaging probe] examination of the brachial artery using the protocol described by the International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilatation of the brachial artery [18]. FMD was measured in response to reactive hyperaemia (endothelium dependent). All participants were studied between 8 and 11 AM after a 12-hour overnight fasting. Any drug known to affect endothelial function e.g. nitrates and aspirin was withdrawn > 1 week before the examination. The measurements were performed in supine position on the non- dominant arm, after 10 minutes resting in quiet, dark room with a temperature of 22°C. The brachial artery was scanned longitudinally 5 - 15 cm above antecubital fossa. The scans were recorded on a super VHS videotape for later measurement of resting diameter and blood velocity. A blood pressure cuff was inflated around the forearm to 40 mmHg above systolic BP of the patient for 4 minutes. Measurement of the maximal diameter was taken 45 - 60 seconds after cuff-release and to serve as a measure of endothelium-dependent vasodilatation reflecting vascular smooth muscle function. All measurements were taken at the end of diastole. Distance measured was from anterior to posterior M lines (media-adventitia interface) and every measurement was taken as an average of 5 consecutive cardiac cycles. The radiologist performing the scan was given only the subject name, no diagnostic information was available. FMD is typically expressed as the change in post-stimulus diameter as a percentage of the baseline diameter. FMD is calculated as: 100% × [(post-deflation diameter - resting diameter) /resting diameter].The obtained results were statistically analyzed by IBM computer using SPSS (statistical program for social science version 12). Description of quantitative variables as mean ± SD and range, description of qualitative variables as number and percentage. Chi-square test was used to compare qualitative variables between groups. Unpaired t- test was used to compare quantitative variables between both groups. Correlation co-efficient test was used to rank different variables against each other positively or inversely. Insignificant; if P > 0.05, significant; P < 0.05.
4. Results
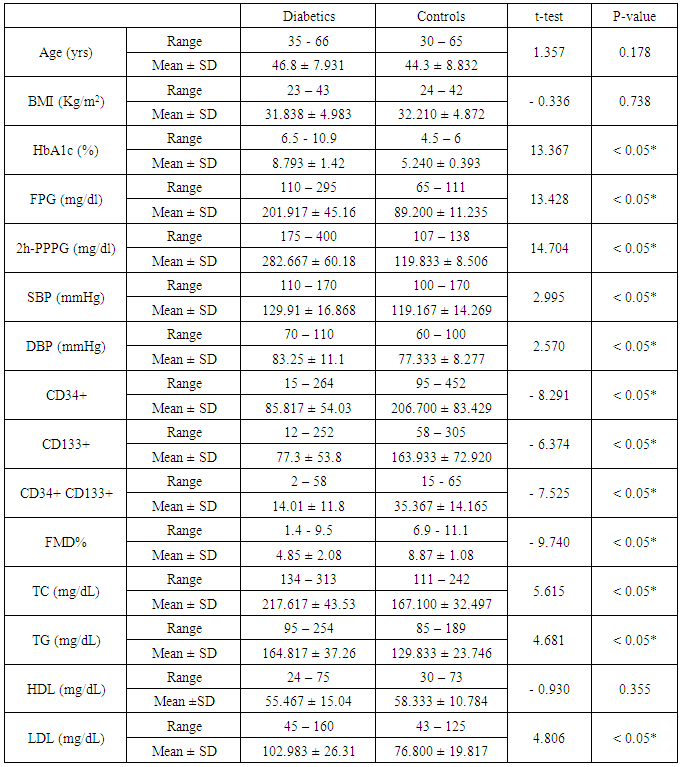
- The study was performed on 2 groups of participants: Group I (patients): 60 patients with T2DM having variable duration of DM, (33 females and 27 males), their age ranged between 35 and 66 years with mean 46.8 ± 7.9 years. Group II (controls): 30 healthy subjects (15 females and 15 males), their age ranged between 30 and 65 years with mean 44.3 ± 8.8 years old. The results obtained were tabulated and statistically analysed in tables (1 - 3).There are lower counts of CD34+, CD133+, and CD34+ CD133+ and impaired FMD% in diabetics compared with controls, however, significant increase of glycemic markers (HbA1c, FPG, 2-h-PPPG), SBP, DBP, and lipid profiles (TC, TG, LDL) in diabetics. No significant difference of BMI and HDL between diabetics and controls was observed (table 1).
|
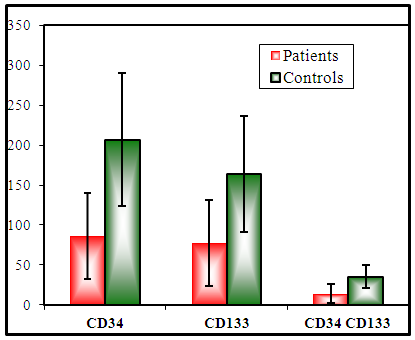 | Figure (1). Histogram of EPCs counts in diabetics and controls. There are significant decrease in CD34+, CD133+ and CD34+ CD133+ counts in diabetics compared with controls |
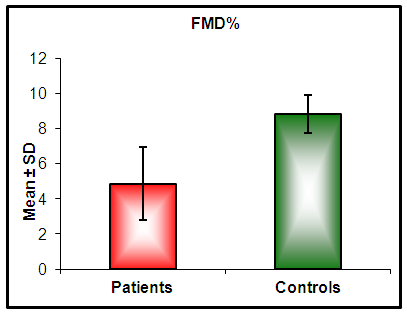 | Figure (2). Histograms of FMD% in diabetics and controls. There is a significant decrease of FMD% in diabetics compared with controls |
|
|
5. Discussion
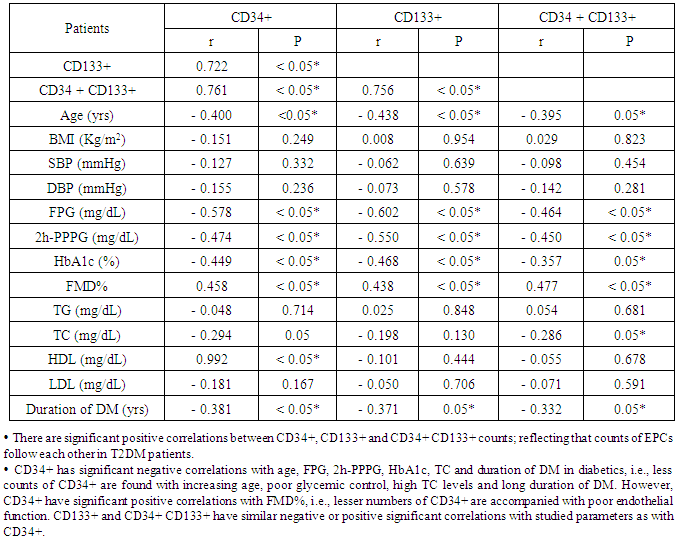
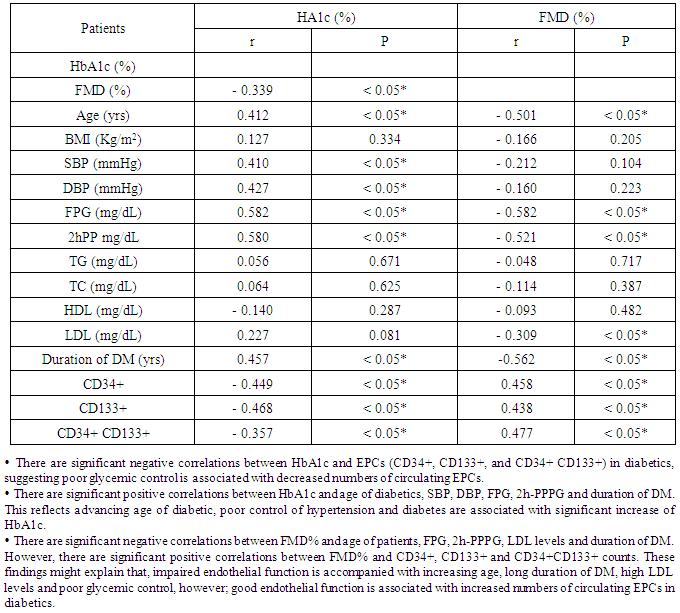
- Diabetes mellitus (DM) affects more than 300 million individuals globally, contributing to significant morbidity and mortality worldwide. The incidence and prevalence of DM continue to escalate with the force of an approaching Tsunami [19]. A source of disability and morbidity of T2DM is related mainly to vascular complications which underlie the development of retinopathy, nephropathy, neuropathy, ischemic heart disease and peripheral vasculopathy [20]. Endothelial dysfunction (ED) is the key etiological factor that induces moderate to severe vascular complications and has been proposed as a key therapeutic target in T2DM patients [21]. Endothelial dysfunction and injury are considered to be the first step in atherogenesis [22]. Many studies indicate that endothelial dysfunction and injury in the vascular wall are repaired by bone marrow (BM)-derived endothelial progenitor cells [EPCs] [23]. An evidence suggests that EPCs are mobilized from the BM into the peripheral blood in response to tissue ischemia or injury [7], these cells migrate to sites of damaged endothelium and differentiate into EPCs [8], thereby improving blood flow and tissue repair [6]. EPCs contribute to re-endothelialisation and neovascularization. Several lines of evidence indicate that EPCs constitutes an important endogenous system that maintains endothelial integrity and vascular homeostasis [6]. Patients with cardiovascular diseases such as, coronary artery disease (CAD), hypertension (HTN), heart failure (HF), DM, exhibit reduced EPCs number and functions [24]. Therefore, reduced EPCs count may reflect a mechanistic link that confers increased risk of adverse cardiovascular outcome. Reversal of EPCs dysfunction could therefore potentially prevent the progression of cardiovascular and vascular disease [25]. The present study was planned to clarify the relationship of circulating EPCs and endothelial dysfunction in T2DM patients. The results of the current work showed that diabetics had significant decrease in EPCs compared with healthy controls (P < 0.05 - Table 1). In diabetics EPCs counts had significant inverse correlations with markers of glycemic control (HbA1c, FPG and 2h-PPPG, respectively; P < 0.05 - Table 2), this means that diabetics with good glycemic control might expected to have more EPCs counts than those with poor glycemic control, implying that glycemic control might affect EPCs counts in T2DM patients. In parallel to the current study results, Fadini et al. [26], who determined the levels of circulating CD34+ and CD34+KDR cells in 219 middle-aged individuals with no pre-diagnosed alteration in carbohydrate metabolism, they found that CD34+ and CD34+KDR cells were significantly reduced in individuals who were found to have T2DM and were negatively correlated with both FPG and 2h-PPPG level in the whole population, while only CD34+ cells but not CD34+KDR cells, were significantly reduced in pre-diabetic individuals. Similarly, Churdchamjan et al. [27], compared EPCs numbers in T2DM patients with good and poor glycemic control and healthy subjects. They found that diabetics had highly significant reduced EPCs numbers [CD34+, CD34+ and VEGFR-2 (vascular endothelial growth factor receptor-2)] than healthy controls. In addition, they found significant inverse correlation between EPCs numbers and HbA1c in the diabetic patients. Moreover, they suggested that, there was EPCs dysfunction in T2DM which might be improved by strict glycemic control but good glycemic control did not reach the level in healthy controls. Also, the present results are in agreement with those reported by Antonio et al. [28] who found that diabetics had reduced circulating numbers of CD34+KDR cells by 63% and CD34+ CD133+KDR EPCs by 50%, when compared with non-diabetics. They also, found significant negative correlations between circulating EPCs and both HbA1c and FPG. In contradictory to the current study results, where there was significant inverse correlation between CD34+ cell numbers and HbA1c level; Hisachi et al. [29] reported insignificant association between circulating CD34+ cell numbers and HbA1c level in T2DM patients. The current study observed that, there was significant inverse correlation between EPCs counts and duration of DM (P < 0.05 -Table 2). Patients with long duration of DM tend to have more reduced EPCs counts. These findings suggested that the duration of DM might have deleterious effect on EPC counts. In agreement with these obtained results, Fadini et al. [30], mentioned that in diabetics with >10 years, the number of CD34+ cells was lesser than reported in those with duration of diabetes < 10 years. In this context, Hisashi et al. [29] showed that the duration of DM had significant inverse association with CD34+ cell number in T2DM patients. They suggested that, a period of high glucose exposure rather than the magnitude of hyperglycemia could contribute to the reduction of circulating CD34+ cell number. Most of the researches study the effect of hyperglycemia on EPCs, but few study the effect of frequent hypoglycemia on EPCs counts in T2DM. For example, Fadini et al. [31] investigated number of CD34+, CD133+ and CD34+KDR in naïve T2DM patients; initiating basal insulin, with and without occurrence of hypoglycemia. They found that, hypoglycemia prevents the increase in vasculoprotective EPCs numbers. Clinically, these data strengthen the importance of avoiding hypoglycemia to improve cardiovascular outcome during the treatment of T2DM. The current study observed that diabetics had significant lower FMD% compared with controls (P < 0.05; Table 1). This reduction of FMD% in T2DM patients was accompanied with similar reduction in EPCs. Also, showed significant positive correlations between EPCs subpopulation and endothelial function in T2DM patients (P < 0.05; Tables 2 and 3). These results were in consistent with Yun-Fei et al. [32] who found significant reduction in number of CD34+KDR in 46 newly diagnosed T2DM that associated also with FMD% reduction. In the same way and in agreement with the obtained results in this work, Kim et al. [3] concluded that, the circulating EPCs number was strongly predictor of FMD. This study found significant negative correlation between serum total cholesterol level and CD34+ and CD34+CD133+ counts (r = - 0.294 and P < 0.05; r = - 0.286; P < 0.05, respectively - Table 2), but insignificant correlation with CD133+ counts (r = - 0.198 and P > 0. 05 -Table 2), this might describe partially the adverse effect of hypercholesterolemia on endothelial function in T2DM which in accordance with mentioned by Thum et al. [33]. In the present work, number of circulating EPCs in diabetics was significantly lower than in controls, which also was associated with impaired endothelial function as measured by FMD%. Some studies were in agreement with these findings, inspite of using another methods for assessment of endothelial function, for example, Chun et al. [5] used 2-dimensional (2D) speckled tracking trans-thoracic echocardiography [special type of echocardiography] for detailed evaluation of left ventricle (LV) functions in T2DM patients, with apparently normal LV dimensions and ejection fraction (EF) with routine echocardiography in 78 patients with T2DM and no history of coronary artery disease. They found that at least 30% of T2DM patients, with apparently normal LV dimensions and EF, have impaired myocardial function. Those with an impaired LV longitudinal strain and circumferential strain, had a lower number of CD34+EPCs (P < 0.05), than those with preserved longitudinal and circumferential strain. Their findings suggested that myocardial dysfunction in patients with T2DM is related to depletion of EPCs. Mauro et al. [34] assessed onset or progression of microangiopathy (endothelial dysfunction) in 187 T2DM patients with a 3.9-years follow-up and whether EPC levels predict onset/progression of microangiopathy. They reported that, new onset or progression of microalbuminuria, chronic kidney disease, retinopathy and neuropathy occurred in 70 patients (9.5%/y) and baseline CD34+ EPCs were significantly lower in patients, these with lower baseline CD34+ or CD133+ EPC levels more likely to experience worsening microangiopathy than those with high cell levels. Also, Maria et al, [35] reported that T2DM patients with vascular foot lesion have less CD34+ than T2DM patients with neuropathic foot lesion. These data could explain why in diabetics neuropathic foot lesion has a better healing progress with respect to ischemic foot lesion. Therefore, the circulating EPC number can be proposed as a biomarker of DM vascular endothelial function and alteration in the circulating EPCs number may also have an important role in the development and progression of endothelial dysfunction in DM. So, ways to reverse EPC alteration in diabetic patients may be useful in improving diabetic vascular endothelial function. Available data suggest that metabolic intervention by either lifestyle change or glucose lowering is able to improve EPC biology. In addition many drugs commonly prescribed in diabetic patients have demonstrated significant EPC modulating effects [36]. The present study was reported that, patients with T2DM had impaired endothelium regenerating capacity as reflected with a decrease in number of circulating EPCs and its association with endothelial dysfunction as measured by FMD%. DM patients who could achieve satisfactory glycemic control had significantly higher circulating level of EPCs and better endothelial function. This might be one of the mechanisms in which satisfactory glycemic control in T2DM patients had reduced cardiovascular events.
6. Conclusions
- Patients with T2DM have close relationship between reduced circulating EPCs number and the degree of endothelial dysfunction regardless presence or absence of other known cardiovascular risk factors. Measurement of the circulating EPCs counts might be applied as a reliable diagnostic tool to identify T2DM patients with impaired endothelial function in asymptomatic one, therefore, alteration of EPCS numbers could be considered as a therapeutic target for early DM vascular protection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML