-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(3): 49-53
doi:10.5923/j.cmd.20160603.02

Diagnostic Performance of Serum Bone Turnover Marker Lysylpyridinoline in Diabetic Foot Osteomyelitis: A Preliminary Study
Oliver G. Hayes , Venkat N. Vangaveti , Usman H. Malabu
Translational Research in Endocrinology and Diabetes [TREAD], College of Medicine and Dentistry, James Cook University, QLD, Australia
Correspondence to: Usman H. Malabu , Translational Research in Endocrinology and Diabetes [TREAD], College of Medicine and Dentistry, James Cook University, QLD, Australia.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objectives: The aim of the study was to determine whether serum levels of lysylpyridinoline (LP) differ between diabetic foot osteomyelitis (DFO) and diabetic foot ulcers without osteomyelitis (cellulitis). Methods: A case controlled study was designed comparing the aforementioned groups. Subjects were classified as osteomyelitis and controls based on the International Working Group diagnostic criteria. Serum LP samples were analysed using enzyme linked immunosorbent assay. Results: The serum LP levels were significantly higher in the DFO group (n: 16) than the control group (n: 11): LP median ± SD of 9.3 ± 3.5 nmol/l, interquartile range (IQR) 6.8-11.2 in DFO compared to 2.1 nmol/l 3.5, IQR 0.7-4.2 in the controls, p=0.001. The receiver operator characteristic curve in the DFO was 0.9. The diagnostic sensitivity, specificity, positive and negative predictive values were 100%, 77.8%, 89.0% and 100% respectively. Conclusions: This appears to be the first evidence of serum LP elevation in diabetic foot osteomyelitis with potential diagnostic value in clinical setting.
Keywords: Bone Turnover Markers, Diagnosis, Diabetic Foot Osteomyelitis
Cite this paper: Oliver G. Hayes , Venkat N. Vangaveti , Usman H. Malabu , Diagnostic Performance of Serum Bone Turnover Marker Lysylpyridinoline in Diabetic Foot Osteomyelitis: A Preliminary Study, Clinical Medicine and Diagnostics, Vol. 6 No. 3, 2016, pp. 49-53. doi: 10.5923/j.cmd.20160603.02.
Article Outline
1. Introduction
- Diabetic foot osteomyelitis (DFO) is a common complication of foot ulcers, with one review indicating rates between 20% and 68% [1]. DFO occurs in 2-3% of all diabetic patients and its chronicity contributes to a poor quality of life associated with pain, suffering and disability [2]. Mortality rates in individuals with DFO have been found to be almost twice as high compared to subjects without diabetes [3]. Diabetic foot bone infection (osteomyelitis) is a leading cause of hospitalisation and lower limb amputation worldwide costing >$40,000 per event [1, 4]. Thus early diagnosis of osteomyelitis may curtail hospitalisation for diabetic foot ulcer, lower limb amputation and death.The current diagnostic modalities for DFO have significant drawbacks due in part to non-availability in rural/remote and underserved healthcare centres. Plain radiography being cheap and readily available has a diagnostic sensitivity of only 54% and a specificity of 68%. Thus, it has poor diagnostic utility, especially at ruling out a diagnosis of osteomyelitis [5]. A Technetium 99m Bone Scan has a higher sensitivity of 81% and dismal specificity of 28%; thus are clinically useful to exclude DFO; however, less so for a positive diagnosis [6]. An indium-111 leukocyte scan has a sensitivity of 74% and a specificity of 68%; thus has equivalent specificity as plain radiography, with increased sensitivity yet available only in highly specialised centres [6, 7]. Magnetic resonance imaging (MRI) on the other hand has the highest sensitivity and specificity of the diagnostic imaging modalities with 90% and 79% respectively, and thus it is considered the gold-standard of the non-invasive diagnostic modalities [6, 8]. However, invasive procedure such as bone biopsy with histology and microbiological culture are considered the absolute gold standard techniques in diagnosing DFO yet the former is rarely performed in routine clinical practice [9]. Overall, the current diagnostic methods for osteomyelitis each have significant concerns including invasiveness (biopsy), extended lag-time to positivity (radiograph), poor sensitivity (plain radiograph) and poor specificity (bone scan). Leukocyte scans, bone scans, MRIs and biopsies all require specialised skills, are costly and have limited availability in resource poor regions [5]. Thus, at present there is no low cost option that has high diagnostic sensitivity and specificity that is available regardless of the level of the health service. The potential value of using lysylpyridinoline (LP) is of particular interest, as this method has not been previously used particularly in resource deprived communities where modern diagnostic facilities are scarce and when available often beyond the reach of an average patient. The diabetic foot ulcer serves as a portal of entry for contiguous spread of microorganisms to the bone [4]. As osteomyelitis being a state of both enhanced bone resorption and bone formation it is proposed that bone turnover markers may be increased in blood circulation [10]. During both bone formation and resorption bone turnover markers are released, and thus it has been theorised that such markers could be of use in the diagnosis or exclusion of diabetic foot osteomyelitis. In spite of this no study on LP, a bone resorption marker, in diagnosis of diabetic foot ulcer despite of its demonstrable value in non-diabetic bone infections [11, 12]. We hypothesised that the DFO group will have significantly higher levels of LP compared to the controls. The aim of the study was to determine serum levels of LP in subjects with diabetic foot osteomyelitis and compare with diabetic foot ulcer without bone infection.
2. Research Design and Methods
- The study was approved by the local hospital’s ethics committee and informed consent was obtained from the participants. Patients with diabetic foot ulcer aged greater than 18 years were included in the study. Subjects were excluded if they have Charcot’s joint or a known metabolic bone or joint diseases including rheumatoid arthritis or gout, osteoporosis or Paget’s disease or renal/hepatic failure. Other exclusion criteria included primary bone cancer or bone metastases. Subjects receiving thiazolidinedione, local or systemic immunosuppressive agents or known osteomyelitis unrelated to a diabetic foot ulcer were also excluded from the study. Foot wound or ulcer was defined as a full thickness lesion involving any portion of the foot or ankle [13, 14]. Wounds characterized as blisters, minor lacerations, or abrasions were excluded from the study. Wound infection was defined clinically, by criteria consistent with the International Working Group on the Diabetic Foot guidelines [9], i.e., the presence of wound purulence or at least two signs or symptoms of local inflammation or systemic symptoms of infection with no other apparent cause. All wounds were evaluated to determine the extent of soft tissue involved (cellulitis) and for any evidence of bone infection (osteomyelitis) [15]. For this study, osteomyelitis cases were defined by the intraoperative histologic findings of osteomyelitis or presence of probe-able bone underlying an ulcer supported by radiological (plain X-ray, magnetic resonance imaging (MRI) or radionuclide scanning) evidence of osteomyelitis in line with standard guideline [8, 9]. Subjects were allocated to the case group if they had findings consistent with osteomyelitis and control group was defined as subjects that met inclusion criteria however did not meet the diagnostic criteria for osteomyelitis (i.e. having cellulitis alone). On entry, detailed history and clinical examination was conducted and a protocol was completed. These included age, sex, diabetes control, site and depth of ulcer, and presence of associated diabetic complications including ischemic heart disease, peripheral vascular disease, peripheral neuropathy (i.e. loss of sensation using Semmes-Weinstein monofilament) and autonomic neuropathy (i.e. postural dizziness, impotence, episodic watery diarrhea and abnormal sweating). Diabetic retinopathy was diagnosed by experienced ophthalmologists based on the retinal images using digital fundus camera after maximal dilatation of the pupils. Morning fasting venous blood samples were taken, which were centrifuged at 3000g for 5 minutes, followed by -80°C storage. Serum haemoglobin a1c was measured by ion exchange high performance liquid chromatography (Beckman Coulter, CA, USA). Serum LP concentration was determined with the My BioSource (San Diego, California, USA) enzyme linked immunosorbent assay kit. The LP kit has a sensitivity of 1.0 ng/ml, recovery average of 99%, intra-assay reproducibility of <15% and inter-assay reproducibility of <15%. Absorption spectroscopy at a wave length of 450nm, in correlation with a line of best fit from standard solutions was used to determine the concentration of LP. The assay was blinded to the investigators.Statistical analysis was performed using Statistical Package for the Social Sciences version 23. The Shapiro-Wilk test was used to determine the normality of the continuous variables. The Students t test was used to analyse parametric continuous variable, and the Mann-Whitney U test was used to determine the non-parametric continuous variable data. Categorical data was analysed using the Chi-squared test. The data of the patients were used to determine the most suitable discriminating concentration of LP. These were calculated according to standard methods [16]. A p value less than 0.05 was predetermined as the cut-off value for statistical significance.
3. Results
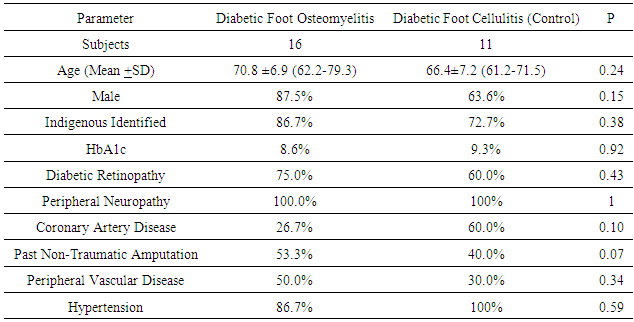
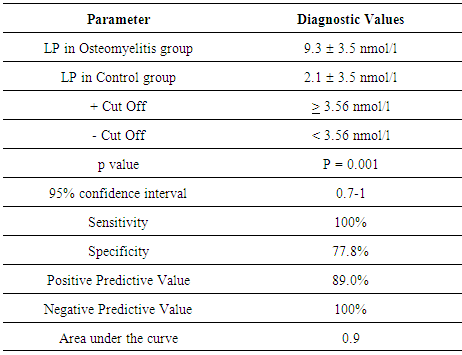
- There were a total of 27 participants, with 11 controls and 16 DFO, thus an enrolment ratio of 1:1.5. The combined demographic and clinical characteristics are shown in Table 1 with a mean age of 67 years, predominantly male, 50% had a previous non-traumatic amputation and the mean HbA1c was 8.2% (66 mmol/mol). The median serum LP was 9.3 3.5 nmol/l, interquartile range (IQR) 6.8-11.2 in the diabetic foot osteomyelitis group and 2.1 3.5 nmol/l, IQR 0.7-4.2 in the diabetic foot ulcer without osteomyelitis group (p=0.001) as depicted in Figure 1. The sensitivity, specificity, positive predictive value and negative predictive value of LP for the diagnosis of diabetic foot osteomyelitis were 100%, 77.8%, 89.0% and 100% respectively as shown in Table 2; receiver operating characteristic (ROC) curve revealed diagnostic value of 0.9 (Figure 2).
|
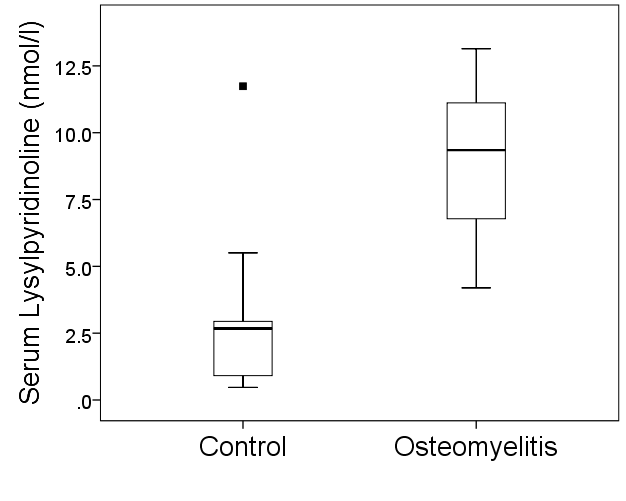 | Figure 1. Serum lysylpyridinoline levels in control group and in subjects with diabetic foot osteomyelitis. Horizontal line representing median, whisker as range and ▪ as outlier |
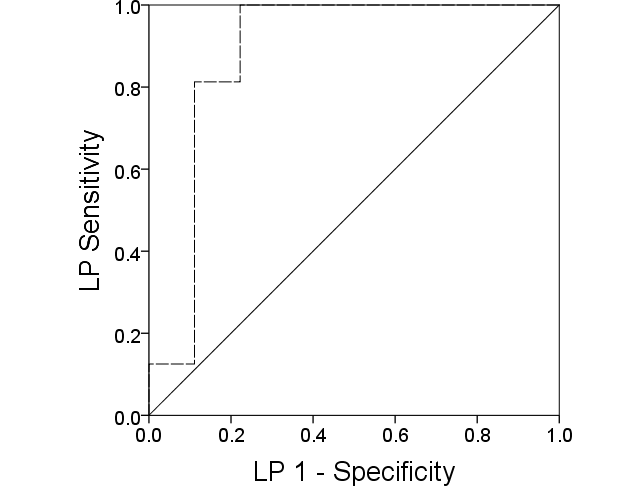 | Figure 2. Receiver operator characteristic curve of serum lysylpyridinoline levels in diagnosis of diabetic foot osteomyelitis demonstrating high diagnostic value |
4. Discussion
- We have demonstrated markedly elevated serum levels of LP in subjects with diabetic foot osteomyelitis with non-overlapping interquartile ranges; thus, there is a statistically and numerically significant difference between osteomyelitis and control groups. The results are in concordance with Shi et al’s investigation of LP in spinal tubercular osteomyelitis and Springer and colleagues’ investigation of LP in mandibular osteomyelitis, both of which showed increased urinary LP in osteomyelitis subjects compared to controls [11, 12]. Our report is novel in that previously it was unknown whether the small volume of bone generally affected in diabetic foot osteomyelitis would raise serum LP. Experimentally induced femoral osteomyelitis in rabbits as reported by Southwood et al could be translated into osteomyelitis of smaller areas of bone in humans with diabetic foot osteomyelitis [17]. The only previous study on bone turnover markers in diabetic foot osteomyelitis was that reported by Nyazee et al showing no significantly raised serum levels of bone formation markers n-terminal telopeptide and bone specific alkaline phosphatase [18]. Thus, the current study, to the authors’ best knowledge, represents the first known report of diagnostic value of LP in diabetic foot osteomyelitis. Using LP in the diagnosis of diabetic foot osteomyelitis has the potential to reduce the dependency on sophisticated diagnostic imaging. The high sensitivity and negative predictive value of LP could in effect stop any negative result from undergoing any of the sophisticated radiological investigations. Thus early diagnosis of osteomyelitis using LP may curtail the need for lower limb amputation, however, confirmatory tests such as biopsy are invasive, accurate diagnostic tests (e.g. MRI) are costly and not readily available in most centres outside major cities. Use of radiographs in detecting osteomyelitis is cheap but has low sensitivity [19, 20]. On the other hand, radioisotope scans are more sensitive than x-rays but are expensive and can be time consuming [7, 21]. We have previously reported use of an inflammatory marker -erythrocyte sedimentation rate (ESR) in diagnosing diabetic foot osteomyelitis [22] but its elevation in other inflammatory and neoplastic conditions limits its widespread use. We believe the increased LP in patients with diabetic osteomyelitis could be due to acute release of the bone resorption turnover marker from infected bone as noted by Nair et al [10]. Our preliminary results showed high specificity and positive predictive values of LP thus indicating a strong ability to rule-in the diagnosis of diabetic foot osteomyelitis. Bone turnover marker testing may have further utility in the rural and remote areas as LP may provide a triaging test to determine whether a suspected DFO should be evacuated to a larger centre for advanced diagnostic imaging. Finally there is a significant cost differential between the Medicare Benefits Scheme fees for LP (17 USD) and advance diagnostic imaging with magnetic resonance imaging and nuclear bone scan costing prohibitively higher, 284 and 235 USD respectively [23]. Our results faced potential limitations including small sample size though it fitted with the outlined objectives of a pilot study. Secondly although combinations of histologic and microbiologic findings are usually considered the gold standard for the diagnosis of osteomyelitis, these procedures may not be routinely performed. Therefore, clinical, laboratory and imaging findings are often used as surrogates for diagnosing osteomyelitis [8]. We used the recommendation of the international working group on the diabetic foot in defining diabetic foot osteomyelitis in this study; basically histologic results and/or combination of clinical and radiological findings consistent with diagnosis of osteomyelitis [9]. Furthermore, histological diagnosis was not conducted on patients with a foot wound in whom there was no suspicion of bone involvement though proved safe [24, 25], we believe it would be unethical to do this procedure on patients with no suspicion of osteomyelitis. In spite of theses our results are consistent with others findings of diagnostic usefulness of bone turnover markers in bone infection [11, 12].In summary, this study has provided first evidence of LP being elevated in diabetic foot osteomyelitis compared to diabetic foot ulcers not complicated by osteomyelitis. Furthermore, our preliminary results suggest possible use of LP in diagnosing diabetic foot osteomyelitis. Finally, with further research LP may provide a viable, low cost, widely available rapid diagnostic methodology for diabetic foot osteomyelitis even in resource-deprived rural health centres.
ACKNOWLEDGEMENTS
- Amy Langley, Julie Goodall, Amelia Turner and Karen Hird for identifying appropriate patients for enrolment and Emily Beric for deciphering the manuscript.
References
| [1] | Lindbloom BJ, James ER, McGarvey WC (2014): Osteomyelitis of the Foot and Ankle: Diagnosis, Epidemiology, and Treatment. Foot and Ankle Clinics. 19(3): 569-88. |
| [2] | Amogne W RA, Amare M (2011): Diabetic foot disease in Ethiopian patients: A hospital based study. Ethiopian Journal of Health Development. 25(1): 17-21. |
| [3] | Iversen MM, Tell GS, Riise T, Hanestad BR, Ostbye T, Graue M, et al. (2009): History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trondelag Health Study, Norway. Diabetes Care. 32(12): 2193-9. |
| [4] | Berendt A, Peters E, Bakker K et al. (2008): Diabetic foot osteomyelitis: a progress report on diagnosis and a systematic review of treatment. Diabetes/Metabolism Research and Reviews. 24: S145-S161. |
| [5] | Dinh MT, Abad CL, Safdar N (2008): Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clinical Infectious Diseases. 47(4): 519-27. |
| [6] | Nawaz A, Torigian DA, Siegelman ES, Basu S, Chryssikos T, Alavi A (2010): Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Molecular Imaging and Biology. 12(3): 335-42. |
| [7] | Mangendre D, Poirier TY (2001): Nuclear medicine in the diagnosis of diabetic foot osteomyelitis. Diabetes Metab. 27: 396-400. |
| [8] | Lipsky BA, Peters EJG, Senneville E et al. (2012): Expert Opinion on the management of infections in the diabetic foot. Diabetes Metab Res Rev. 28(Suppl 1):163–178. |
| [9] | Lipsky BA, Aragón-Sánchez J, Diggle M, Embil J, Kono S, Lavery L, et al. (2016): International Working Group on the Diabetic Foot guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 32 Suppl 1:45-74. |
| [10] | Nair SP, Meghji S, Wilson M et al. (1996): Bacterially induced bone destruction: mechanisms and misconceptions. Infection and Immunity. 64: 2371-2380. |
| [11] | Shi J, Wang Z, Li H, Yuan H (2012): Diagnostic performance of the urinary deoxypyridinoline in spinal tuberculosis. Orthopedics. 35: e922-926. |
| [12] | Springer ING, Wiltfang J, Dunsche A et al. (2007): A new method of monitoring osteomyelitis. International Journal of Oral and Maxillofacial Surgery. 36: 527-532. |
| [13] | Armstrong DG, Harkless LB (1998): Outcomes of preventative care in a diabetic foot specialty clinic. J Foot Ankle Surg. 37: 460-466. |
| [14] | Oyibo SO, Jude EB, Tarawneh I et al. (2001): A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care. 24: 84–88. |
| [15] | Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. (2014): The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 59(1): 220-34.e1-2. |
| [16] | Galen RS, and Gambino SR (1975): Beyond normality the predictive value and efficiency of medical diagnosis. New York: John Wiley. |
| [17] | Southwood LL, Frisbie DD, Kawcak CE, McIlwraith CW (2003): Evaluation of serum biochemical markers of bone metabolism for early diagnosis of nonunion and infected nonunion fractures in rabbits. American Journal of Veterinary Research. 64: 727-735. |
| [18] | Nyazee HA, Finney KM, Sarikonda M et al. (2012): Diabetic foot osteomyelitis: bone markers and treatment outcomes. Diabetes Research and Clinical Practice. 97: 411-417. |
| [19] | Wheat J. Diagnostic strategies in osteomyelitis (1985): Am J Med 78: 2181-24. |
| [20] | Newman LG (1995): Imaging techniques in the diabetic foot. Clin Podiatr Med Surg 12: 75-86. |
| [21] | Rosenthal L (1992): Radionuclide investigation of osteomyelitis. Curr Opin Radiol 4: 62-69. |
| [22] | Malabu UH, Al-Rubeaan KA, Al-Derewish M (2007): Diabetic foot osteomyelitis: usefulness of erythrocyte sedimentation rate in its diagnosis. West Afr J Med. 26(2): 113-116. |
| [23] | Australian Government DoH (2014): Medicare Benefits Schedule Book Category 5 In. Canberra. |
| [24] | Senneville E, Melliez H, Beltrand E et al. (2006): Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: concordance with ulcer swab cultures. Clin Infect Dis 42: 57–62. |
| [25] | Kessler L, Piemont Y, Ortega F et al. (2006): Comparison of microbiological results of needle puncture vs. superficial swab in infected diabetic foot ulcer with osteomyelitis. Diabet Med 23: 99–102. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML