-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(2): 21-31
doi:10.5923/j.cmd.20160602.01

Laminin and Chromogranin A as Serum Markers of Liver Fibrosis in Hepatocellular Carcinoma
Wafaa Mohi El-Deen Abd El-Fatah 1, Mona Abd El-Raof Abd El-Kader 2, Mohammed Taha Abdel-Hak 3
1Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3Department of Radio-Diagnosis, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Wafaa Mohi El-Deen Abd El-Fatah , Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cirrhosis of the liver is the most important risk factor for hepatocellular carcinoma (HCC), which represents the leading cause of death in these patients. The most useful serum marker of the tumor, alpha-fetoprotein (AFP), has a highly variable sensitivity and specificity in the reported studies. Laminin (Ln) is a glycoprotein which has an important role in the mechanism of fibrogenesis and is, thus, related to hepatic fibrosis in addition to presenting increased levels in several types of neoplasias. Elevated Chromogranin-A(CgA) in patients with cirrhosis and superimposed HCC suggesting that raising CgA levels, likely due to a neuroendocrine component of the tumor, might be a useful prognostic marker for HCC in cirrhotic patients. Aim of the work: The objective of the present study is to determine the relationship between laminin and chromogranin- A in plasma of patients with hepatocellular carcinoma in order to determine whether laminin and chromogranin- A may serve as markers for liver fibrosis and whether these parameters could be correlate with liver functions of these patients Subjects and methods: This study was conducted on 50 Egyptian subjects and classified into two groups: Thirty patients of hepatocellular carcinoma (HCC) with previous history of hepatitis C group(G II) (n = 30), in addition to twenty age and sex matched healthy individuals as a control group(GI). HCC was diagnosed histologically or by imaging. They were recruited from the outpatient clinic of Internal Medicine Department of Al-Zaharaa University Hospital, Cairo, Egypt. Liver function tests (AST, ALT, GGT, total bilirubin and albumin) by colorimetric assay, Plasma Laminin and chromogranin-A (by ELISA) and alpha fetoprotein (by immunometric assay) were estimated. Results: laminin and CgA levels were significantly higher in group II (HCC patients) when compared to group I (control subjects) (p<0.0001). Statistically significant positive correlation between CgA versus Ln at HCC group (p< 0.0001). Statistically significant positive correlation between CgA versus GGT (p=0.001) and Ln versus GGT at HCC group (p< 0.0001). CgA level shows best cut off 106 (ng/ml) and Ln level shows best cut off 1065 (ng/ml) and CgA and Ln levels shows %100 sensitivity and %100 specificity in HCC group, which proof that both markers are considered high validity tests in prediction of HCC and hepatic cirrhosis. Conclusions: Laminin and CgA represent useful diagnostic as well as prognostic biomarkers for liver cirrhosis and HCC.
Keywords: Laminin, Chromogranin A, Liver cirrhosis and hepatocellular carcinoma
Cite this paper: Wafaa Mohi El-Deen Abd El-Fatah , Mona Abd El-Raof Abd El-Kader , Mohammed Taha Abdel-Hak , Laminin and Chromogranin A as Serum Markers of Liver Fibrosis in Hepatocellular Carcinoma, Clinical Medicine and Diagnostics, Vol. 6 No. 2, 2016, pp. 21-31. doi: 10.5923/j.cmd.20160602.01.
1. Introduction
- Hepatocellular carcinoma (HCC) is the commonest primary cancer of the liver [1]. While the incidence of HCC has reportedly risen over the last 5-8 years, the survival of those affected has not changed significantly in the last two decades [2]. This is related to both its late detection the lack of effective therapies for advanced stage disease [3]. In Egypt, between 1993 and 2002, there was an almost two folds increase in HCC amongst chronic liver patients [4].Up to 80% of HCCs develop against a background of cirrhosis of the liver and the surveillance of the at risk cirrhotic population could aid earlier detection of the disease and decrease the cancer related mortality rate, the present success is limited by the lack of sensitive biomarkers. Currently, standard surveillance includes a combination of 6 monthly abdominal ultrasound scan (USS) and serumAlphafetoprotein (AFP) measurement, but this strategy does not reliably detect early disease. The diagnostic performance of AFP is inadequate as it is only elevated in 40-60% of cases [5, 6], while abdominal USS is difficult in cirrhotic nodular livers and notoriously user dependent [7]. Alternative serum biomarkers are being actively sought and proposed candidates include Laminin (Ln) and chomogranin-A Laminin is one of the main glycoproteins of the basement membrane (BM) and participates in a series of such biological phenomena as adhesion, migration, cellular differentiation and growth, inflammatory response and the maintenance of the cytoskeleton upon its binding to several components of the matrix, such as collagen type IV, heparan-sulphate and entacin [8]. It has an important role in the mechanism of fibrogenesis and is, thus, related to hepatic fibrosis in addition to presenting increased levels in several types of neoplasias [9]. In the liver, cancer cells do not lie on a BM structure, as other epithelial tumor cells do, but live in trabecular structures surrounded by a tissue enriched by extracellular matrix (ECM) proteins secreted as a consequence of the underlying cirrhosis [10]. Therefore, HCC cells need to penetrate through these ECM tissue boundaries to spread to surrounding or distant sites. However, the mechanisms underlying this cellular invasiveness are not fully understood [11]. The development of direct non invasive markers of hepatic fibrosis is closely linked to pathophysiological understanding of fibrogenesis [12]. Pathophysiologically, along with the pathological process of liver fibrosis, balance between synthesis and degradation of extracellular matrix in liver tissue (ECM) is disturbed, leading to excessive proliferation and abnormal deposition of ECM, which is reflected by release into serum of ECM components/ degraded products, collagenases and certain cytokines [13]. Lns are mainly expressed in vessel wall, bile duct and lymphatic wall, but little in normal hepatocytes [13]. They are over-expressed in liver fibrosis and deposited in sinusoidal endothelial cell gap, which reduces permeability of endothelial cells, and consequently leading to increased portal pressure. Elevated Ln level indicates fibrosis of the sinusoidal capillaries and portal triad [14]. Not only have serum laminin levels been studied in patients with liver diseases, but also in patients with cancer, especially in cases where tumor proliferation and invasion are found [15]. Due to this relationship between laminin tissue deposition and advanced fibrosis, serum levels of laminin have been used by several authors as a non-invasive parameter to assess liver fibrosis in alcoholic patients as well as in those presenting with viral hepatitis and hemochromatosis [16]. Hepatocellular carcinoma commonly exhibits histological polymorphism even within a single nodule. The neuroendocrine character has been observed in some tumor cells within some HCC nodules and elevated serum chromogranin A (CgA) also been reported in patients with HCC [17].Chromogranin-A (CgA) is a 50-kμ acid glycoprotein. It is present in low concentration in the serum of healthy individuals. It has a wide distribution in secretory vesicles of the endocrine, neuroendocrine and nervous system, where it is co-stored and co-secreted with hormones and neurotransmitters [18]. The activation of endocrine system in cirrhosis and HCC is supported by oversecretion of peptides and hormones. Moreover, high-plasma CgA may be due to a great number of biological events, including impaired degradation mechanisms other than increased production. On the other hand, the correlation existing between plasma CgA and degree of liver fibrosis suggests that CgA excretion may be involved during chronic hepatitis [19].HCC showed the highest levels of CgA, up to 30 times the upper limit of normal [19]. This finding suggests that determination of CgA serum values is useful in monitoring patients with cirrhosis of the liver for early detection of HCC.
2. Subjects and Methods
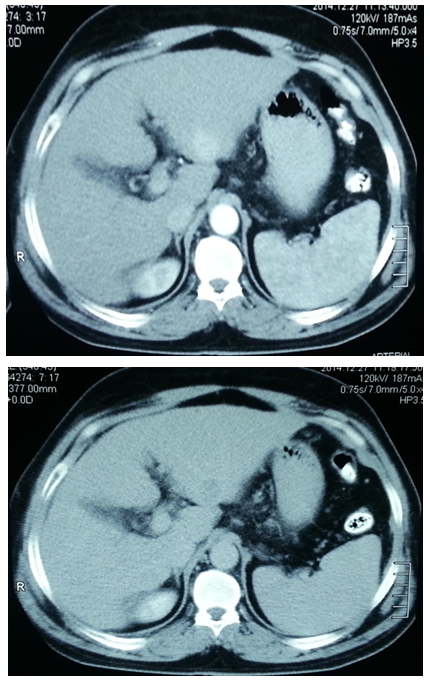
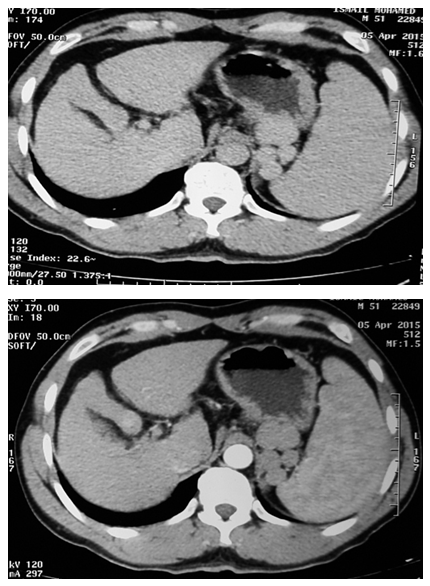
- This study was conducted on 50 subjects with their ages ranging between 35-65 years. They were classified into 2 groups: Group I: included 20 healthy normal-noncirrhotic control subjects. They were 11 females and 9 males with their ages ranging between 35-60 years, recruited consecutively among physicians and coworkers. Group II: included 30 HCC patients, all with previous history of hepatitis C. They were 12 female and 18 male patients with their ages ranging between 43-65 years. Patients were recruited from the outpatient clinic of Internal Medicine Department of Al-Zaharaa University Hospital, Cairo, Egypt. All studied individuals included in this study were evaluated by history taking, thorough clinical examination, and laboratory tests including:a) Liver function tests: -Plasma Alanine Aminotransferase (ALT): Kits provided by (dp International. Jaipur, Rajasthan state, India) were used. Colorimotric determination of ALT activity according to [20].- Plasma Aspartate Amino transferase (AST): Kits provided by (dp International. Jaipur, Rajasthan state, India) were used. Colorimetric determination of AST activity according to [20]. - Plasma Gama Glutamyl Transferase (GGT): Kits provided by (BioSystems S.A. Costa Brava 30, Barcelona, Spain) were used [21]. - Plasma Total Bilirubin: Kits provided by (Diamond Diagnostics. Fisk Streat Holiston, MA, USA) were used [22].- Plasma Albumin: Kits provided by (Diamond Diagnostics. Fisk Streat Holiston, MA, USA) were used [23].b) Plasma laminin by ELISA. Kit provided by (Takara Bio Ink. Seta 3-4-1, Otsu, Shiga, Japan) were used [24]. c) Plasma Chromogranine A by ELISA. Kits provided by (Epitope Diagnostics Inc. San Diego, California, USA) were used [25].d) Alpha fetoprotein (AFP) by immunoenzymometric assay. Kits provided by (Monobind Inc. Lake Forest, Ca 92630, USA) were used [26].In addition toradiological investigations for diagnosis of HCC was based on one of the following: Ultrasound and computed tomography (CT) (Figures 1, 2) with elevated AFP of more than 400 ng/ml, abdominal triphasic computerized tomography (CT) scan and histopathological assessment if needed. Other malignancies or autoimmune diseases were excluded from the study. A verbal informed consent was obtained from all subjects enrolled in the study. Specimens Collection: Ten ml of blood were collected from antecubital vein by venipuncture. Blood samples were collected in fluoride containing tubes for obtaining blood plasma. Plasma samples were divided into several aliquots and stored at -20°C until used for measurements of laminin, Chromogranin A, ALT, AST, GGT, total bilirubin, albumin and alpha fetoprotein. Analysis of data was done by IBM computer using SPSS (statistical program for social science version 16) [27]. Data was summarized using mean, standard deviation and rang for quantitative variables. Comparison between Two groups was done using independent sample t-test. Correlations were done to show the relation between quantitative variables. Laminin and Chromogranine A cut-off level with best sensitivity and specificity was done to show how well laminin and Chromogranine A result reflects HCC. p-values were considered as statistically significant.P value >0.05 insignificantP<0.05 significantP<0.001 highly significant
3. Results
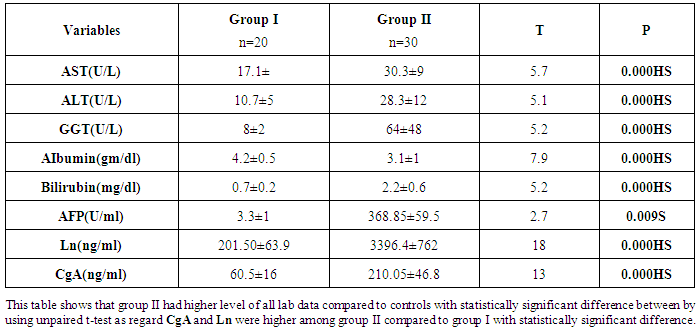
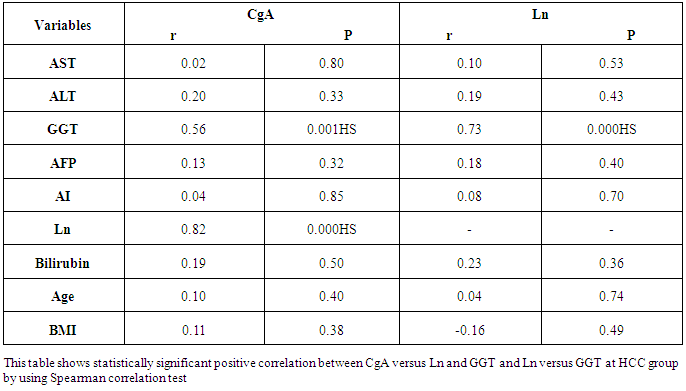
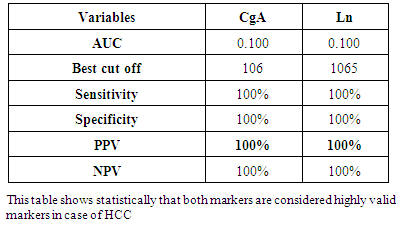
- Results are summarized in tables 1 to 4 and figures 3 to 7. It revealed the following:By using unpaired t-test:Ÿ No statistically significant difference between the studied groups as regard general data (gender, age, BMI) (P >0.05) (table 1).Ÿ HCC group(Group II) had higher level of ALT, AST, GGT, albumin and bilirubin compared to controls with statistically highly significant difference p < 0.0001 (table 2).Ÿ CgA and Ln were higher among group II compared to group I with statistically highly significant difference p< 0.0001 (table 2 & Fig. 3, 4).Ÿ AFP was higher among group II compared to group I with statistically significant difference p=0.009 (p<0.05) (table 2).By using Spearman correlation test:Ÿ Statistically significant positive correlation between CgA versus GGT (P=0.001) and Ln versus GGT (P< 0.0001) at HCC group by using Spearman correlation test (table 3 & Fig.5).Ÿ Statistically significant positive correlation between CgA versus Ln at HCC group P< 0.0001 (table 3 & Fig.6)By using ROC curve:Ÿ CgA level shows best cut off 106 (ng/ml) and Ln level shows best cut off 1065 (ng/ml) ((table 4)Ÿ CgA and 03Ÿ Levels shows%100 sensitivity and%100 specificity in HCC group (table 4 & Fig.7), which proof that both markers are considered high validity tests in prediction of HCC and hepatic cirrhosis.
|
|
|
|
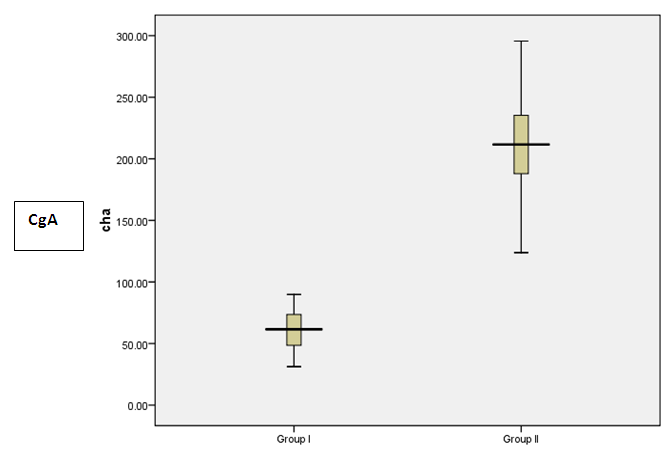 | Figure 3. Box plot to show the difference between the studied groups as regards CgA |
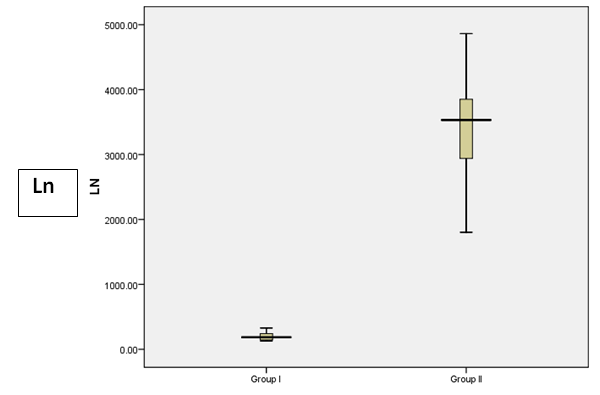 | Figure 4. Box plot to show the difference between the studied groups as regards Ln |
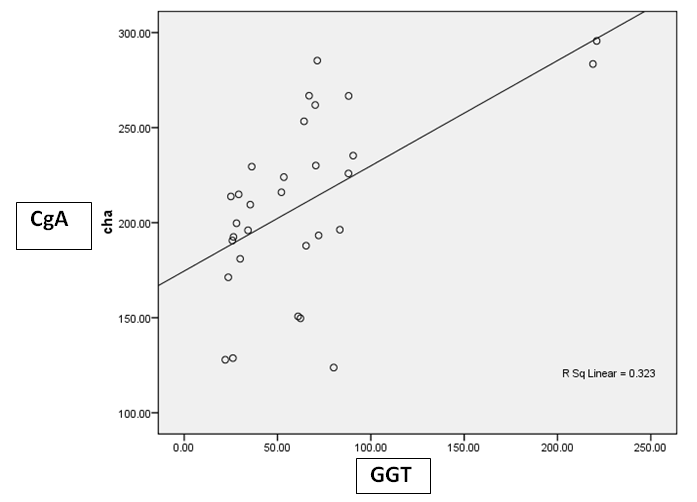 | Figure 5. Correlation between CgA versus GGT at HCC group |
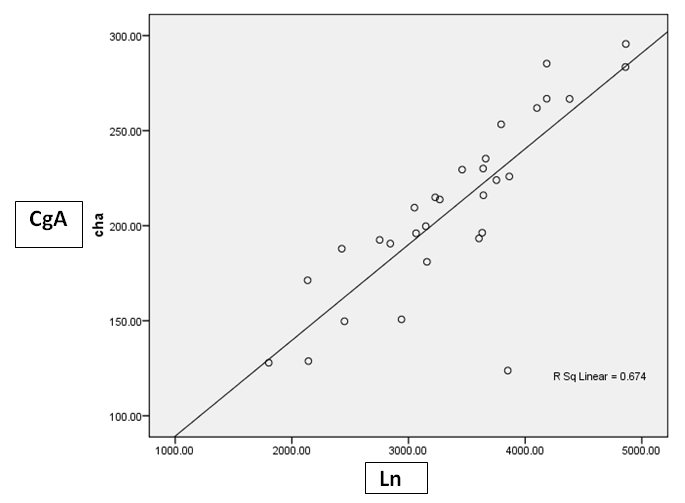 | Figure 6. Correlation between CgA versus Ln at HCC group |
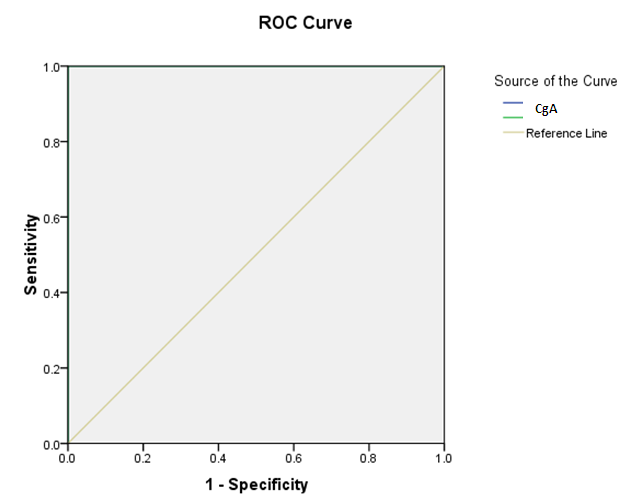 | Figure 7. |
4. Discussion
- Worldwide, the patients suffering from primary liver cancer are commonly complicated with cirrhosis, with a prevalence the latter of 72.1% to 82.3%. Compared to non-cirrhotic ones, the cirrhotic patients have relatively poor liver regeneration ability and impaired functional reservation [13].On the other hand, Up to 80% of HCCs develop against a background of cirrhosis of the liver [4]. Campbell and coworkers reported that liver biopsyis regarded as the historical ‘gold standard’ for diagnosis and assessment of prognosis in chronic liver diseases [28]. However, other study stated that liver biopsy with its associated life threatening risks and morbidity make it a poor choice when considering assessment of liver fibrosis progression or regression. Furthermore, there is the issue of sampling error, defined as variable levels of fibrosis throughout the liver, with biopsy only examining a small (1/50,000) portion of the liver [29].Therefore, non-invasive assessment methods for liver fibrosis have been extensively investigated. Recently, blood biochemical markers exhibited considerable correlation with liver fibrosis and several predictive models were developed. The development of direct noninvasive markers of hepatic fibrosis is closely linked to pathophysiological understanding of fibrogenesis [12].Moreover, Cales and coworkers reported that a single marker cannot be as efficient as a combination of markers in predicting significant liver fibrosis [30].Laminin is a glycoprotein which has an important role in the mechanism of fibrogenesis and is, thus, related to hepatic fibrosis in addition to presenting increased levels in several types of neoplasias [9]. With the development of hepatic cirrhosis, laminin and collagen deposition occurs both along the fibers of septal fibrosis and subendothelial sinusoids or Disse’s space. At the latter site, laminin deposition, together with collagen deposition, determine the formation of a true basement membrane along sinusoids. This phenomenon is called capillarization of Disse’s space [31]. Chromogranin-A (CgA) is a 50-kμ acid glycoprotein originally described in catecholamine storage vesicles of the adrenal medulla [18]. Clusters of cells containing CgA have been demonstrated within HCC tissue [32]. One study, reporting high serum CgA values in approximately half patients with HCC [33]. Massironi and coworkers confirm the presence of elevated plasma levels of CgA in both patients with chronic liver disease [chronic hepatitis and liver cirrhosis (LC) and those with hepatocellular carcinoma (HCC). Though HCC showed the highest levels of CgA, up to 30 times the upper limit of normal [19]. This finding suggests that determination of CgA serum values is useful in monitoring patients with cirrhosis of the liver for early detection of HCC.In the current study, the results revealed that, levels of laminin were significantly higher in group II (HCC patients) when compared to group I (control subjects) (p<0.0001). This is consistent with Gao and his colleagues who reported that, serum laminin concentration is increased in metastatic cancer of different origins such as melanoma, gastric adenocarcinoma, hepatocellular carcinoma, colorectal cancer and epithelial ovarian tumor [34].In concomitant with the results, another experimental study which reported that values of serum laminin and galectin-3 in HCC rats were significantly higher than those in normal rats [35].Moreover, Catarino and coworkers who reported that the mean serum laminin values were significantly higher in patients with cirrhosis than in individuals from the control group [36]. Data from many studies reported that serum laminin concentrations increase in chronic liver disease [18, 37, 38]. In addition, other searchers reported that laminin concentrations increase in early stages of chronic liver disease and the highest concentrations were in active cirrhosis and chronic active hepatitis [39, 36].Moreover, many other studies whoreported that patients with higher stages of liver fibrosis had higher serum laminin (Ln) concentration [40, 41, 42]. Neves and coworkers proved that the increased serum laminin concentration has been related to hepatic fibrosis and liver function in both human and experimental studies [43].In addition, Ratziu and coworkers who reported that laminin determination could be used for the screening of patients to be submitted to a liver biopsy, since it presents a better performance than the criteria usually used to indicate a biopsy for these patients [44]. Also, another study had proposed laminin serum concentrations as a sensitive screening test for hepatic fibrosis and portal hypertension in chronic hepatitis C [45].The elevation of plasma laminin concentrations in HCC patients might be explained by the formation of basement membranes in the hepatic sinusoids [36]. As, Smedsrod and coworkers reported that the increased production of laminin in the liver, and lack of degradation of this protein by liver endothelial cells should also be considered [46]. Apart from an increase in tissue deposition or turnover, there would be a decrease in the liver’s ability to degrade this protein. With the development of anti-laminin antibodies, directed against the laminin P1 portion, increased levels of this circulating protein were observed in the more advanced stages of fibrosis in patients with hepatic disease [47].The results revealed that plasma CgA was significantly higher in group II (HCC patients) when compared to group I (controls) (P< 0.0001).These results are consistent with other study which reported that the HCC patients showed a significant increase in CgA (p<0.001) compared to controls [48].The results were in agreement with the results obtained from Salama and others, and Spadaro and coworkers who reported a statistically significant elevation of CgA serum levels in cirrhotic patients when compared to healthy subjects (P< 0.001). Data from other researchers reported that elevated levels of plasma CgA are found in cirrhotic patients [19]. The fact that plasma CgA levels are higher than normal in the early status of chronic liver disease suggests this protein could be secreted through an activation of endocrine cells during hepatitis and not necessary reflect reduced CgA catabolism due to progressive hepatocellular failure [19]. Moreover, Leone and his colleagues reported elevated CgA serum values in 43% of patients with cirrhosis and superimposed HCC suggesting that raising CgA levels, likely due to a neuroendocrine component of the tumor, might be a useful prognostic marker for HCC in cirrhotic patients [33]. However, high serum CgA values are also found in patients with hepatic failure, CRF and CHF, possibly because of inadequate hepatic metabolization, reduced renal elimination and neuroendocrine activation [18]. Circulating CgA levels in HCC patients could reflect CgA expression in HCC tissues [50], however elevated levels of plasma CgA are found also in HCC-free cirrhotic patients, as expression of the endocrine phenotype of both cirrhotic and HCC cells, following histologic [51] and scintigraphic demonstration of somatostatin receptors on liver cell membranes [52]. HCC group (Group II ) had higher level of ALT, AST, GGT, albumin and bilirubin compared to controls with statistically highly significant difference (p<0.0001). AFP was higher among group II compared to group I with statistically significant difference (p=0.009) .This is consistent with another study which reported that there was a statistically highly significant elevation of serum AFP (P<0.001) in HCC group when compared to control group [53]. Moreover, Lin Zhou and others, who reported that, serum alpha fetoprotein (AFP) is widely used as a tumor marker in detecting patients with hepatocellular carcinoma, and has been proven to have capability of prefiguring the prognosis [54]. In addition, another study reported that, testing for AFP serum levels is a useful tool for early detection and diagnosis of HCC [55].This study also showed that GGT had significantly higher mean level in group II when compared to group I (p<0.0001), The result in agreement with Forns and coworkers who reported that GGT is a marker of liver fibrosis and its prognostic value was independent from the bilirubin and transaminase levels [56]. Increased GGT values may be explained by HCC causes bile duct damage and steatosis. Bile duct lesions can partially explain increased GGT values and it appears that patients with bile duct lesions have significantly higher fibrosis scores [57].In the current study, plasma laminin and Chromogranin A were significantly positively correlated with GGT in HCC patients (P<0.001). This may be explained by the presence of fibrosis in HCC patients, which is associated with increased serum GGT and this is consistent with a study which reported that GGT is a marker of liver fibrosis and its prognostic value was independent from the bilirubin and transaminases levels [56].In the current study, plasma laminin and Chromogranin A were positively significantly correlated with each other (p < 0.0001). Cales and coworkers reported that a single marker cannot be as efficient as a combination of markers in predicting significant liver fibrosis [30].In the current study Chromogranin A was found to be non-significantly correlated with AFP. These results are consistent with other studies which reported that no significant correlation was found between AFP values and CgA values in patients with a cirrhosis (P=0.50) [18, 49] Furthermore, Spadaro and co-workers reported that values of AFP were not correlated with CgA values. Therefore, when AFP is normal or <200 ng/mL and in the presence of suspicious clinical, laboratory and/or imaging signs of HCC, the evaluation of CgA levels becomes of particular importance in the follow-up of chronic liver disease patients [18].These results were in disagreement with the results obtained from Massironi and others who reported that CgA and AFP levels correlated significantly in the whole group of patients with chronic liver disease (p < 0.0001) [19]. In the present study, by using a cut-off point =1065 ng/ml of laminin the validity of plasma laminin as a predictor for HCC patients showed a sensitivity of 100%, a specificity of 100%, positive predictive value of 100%, negative predictive value of 100% and overall accuracy value of 100% for the diagnosis of hepatocellular carcinoma. Mosa and other researchers reported that by using a cut-off point=48U/L for Laminin, sensitivity for diagnosing fibrosis was found to be 88%, specificity=63.6% and accuracy=80.6% [58]. These results are consistent with another study which reported that as regarding serum laminin concentrations in patients with liver fibrosis, the sensitivity was 96.8%, the specificity was 80%, the positive predictive value was 93.7%, the negative predictive value was 88.8% [41]. Laminin presented the best diagnostic performance, with 87% accuracy [59].Furthermore, other researchers reported that for serum laminin in cirrhotic group values higher than 2.25 μg/ml, the diagnostic sensitivity was 100% and the specificity 73% and ROC curve analysis showed that the area under the curve of serum laminin was 0.967 (P < 0.01) [36].In the present study, the diagnostic performance of plasma chromogranin A showed a sensitivity of 100%, a specificity of 100%, positive predictive value of 100%, negative predictive value of 100% and overall accuracy value of 100% for the diagnosis of HCC. ROC curve was performed to show the diagnostic value of chromogranin in diagnosis of HCC patients taking a cutoff level 106 ng/ml. These results are consistent with Spadaro and coworkers who determine the sensitivity and the specificity for HCC detection, they constructed a range of CgA values in HCC and assumed as cut-off in the lower value of the range (250 ng/mL). This cut-off appears to have a good sensitivity and specificity, respectively 61% and 82%, for detection of HCC in patients with liver cirrhosis [18]. Other researchers reported that when using the ROC curve to improve the specificity and sensitivity of Cg-A, the cutoff value of 16.75 nmol/l yielded a sensitivity and specificity of 66.7% and 80% respectively (best cutoff). The are a under the curve (AUC) for Cg-A was 0.851 [49]. Moreover, Spadaro and coworkers concluded that a follow-up of CgA serum values after treatment of HCC is recommended in order to define the utility of the marker for the detection of recurrent tumor [18].
5. Conclusions
- Usage of liver biopsies has several technical limitations and complications. Combination of markers are of great value not only in patients at risk for liver biopsy, but also as a part of the assessment of patients with chronic liver disease, avoiding the invasive methods. Laminin and CgA represent useful diagnostic as well as prognostic biomarkers for liver cirrhosis and HCC.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML