-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2016; 6(1): 13-19
doi:10.5923/j.cmd.20160601.03

Evaluation of Sensititre® MYCOTB Panel for the Susceptibility Testing of Mycobacterium tuberculosis to First and Second Lines Anti-Tuberculosis Drugs
Safaa M. Abdel-Rahman1, Dina Erfan1, Walaa Abdel-Latif1, Hoda Kholeif2
1Department of Medical Microbiology and Immunology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
2Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Hoda Kholeif, Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: With the global rise in Mycobacterium tuberculosis (Mtb) resistance, there is a need for a simple, rapid, quantitative susceptibility method for testing Mtb strains. The reference method for testing Mtb susceptibility is Agar Proportion (APM), which is a one or two-concentration breakpoint test. The Sensititre MYCOTB MIC plate (MYCOTB) is a micro titer plate containing lyophilized antibiotics and configured for determination of MICs to first and second line anti-tuberculosis drugs. The objective of this study was to compare the Sensititre MYCOTB plate (MYCOTB TREK Diagnostic Systems) for susceptibility testing of Mycobacterium tuberculosis (Mtb) to the modified agar proportion method (APM). Materials and methods: Forty clinical isolates from sputum specimen of tuberculosis patients were selected (multidrug resistant and extensively drug resistant strains) and obtained from Myco bacteriology Central lab- Egyptian ministry of health. These strains were subcultured on Lowenstein-Jensen media. Antibiotic susceptibility was carried to first and second line anti tuberculosis drugs (isoniazid, rifampin, ethambutol, ethionamide, cycloserine, kanamycin, streptomycin, amikacin, ofloxacin, rifabutin, moxifloxacin and para-aminosalicylic acid) by agar dilution method as a reference method and the Sensititre MYCOTB minimal inhibitory concentration (MIC) plate (MYCOTB). Result of Sensititer plate was expressed as sensitivity, specificity and categorical agreement. Results: No statistical significant difference was found between the results of Sensititre plate and APM regarding any of first or second line anti-tuberculosis drugs except with cycloserine P-value = 0.0003. The highest sensitivity of Sensititer plate was to isoniazid 0.2μg/ml, 1μg/ml and ofloxacin 100%. While the least sensitivity for detection of resistance was to moxifloxacin 40%. The highest specificity was to kanamycin, ofloxacin and amikacin 96%, 95.65% and 92.86% respectively. The lowest specificity was to isoniazid 1μg/ml 14.29% and cyclosiren 36.67%. Categorical agreement was highest for isoniazid 0.2μg/ml and ofloxacin (97.5%). The lowest categorical agreement was for cycloserine (45%). Optimum growth for Sensititer MYCOTB plate readings was between 10 and 14 days, whereas the APM read times were days 14 and 21. In conclusions: The Sensititer MYCOTB plate does not need instrumentation for interpreting the results and yielded results in shorter time than APM with advantage of including second line anti-tuberculosis drugs in the same plate. Concordance was high between APM and the MYCOTB plate except for cycloserin and ethambutol in concentration 10μg/ml. Sequencing is needed to identify the cause of discrepant results for these two antibiotics.
Keywords: MDR-TB, XDR-TB, Sensititre MYCOTB MIC plate (MYCOTB), APM
Cite this paper: Safaa M. Abdel-Rahman, Dina Erfan, Walaa Abdel-Latif, Hoda Kholeif, Evaluation of Sensititre® MYCOTB Panel for the Susceptibility Testing of Mycobacterium tuberculosis to First and Second Lines Anti-Tuberculosis Drugs, Clinical Medicine and Diagnostics, Vol. 6 No. 1, 2016, pp. 13-19. doi: 10.5923/j.cmd.20160601.03.
Article Outline
1. Introduction
- TB now ranks alongside HIV as a leading cause of death worldwide. HIV’s death toll in 2014 was estimated at 1.2 million, which included the 0.4 million TB deaths among HIV positive [1].Anti-tuberculosis (TB) drug resistance is a major public health problem that threatens progress made in TB care and control worldwide [2]. In developing countries, Multidrug resistant (MDR) and extensively drug-resistant (XDR) strains can represent up to 40% of all new cases [3].Multidrug resistant (MDR) strains are resistant to isoniazid and rifampin. Extensively drug-resistant (XDR) strains are resistant to isoniazid, rifampin, to at least one quinolone, and at least one of the second-line injectable drugs (kanamycin, amikacin, or capreomycin). Lately pan resistant strains, resistant to all first and second line drugs with a proven activity against M.tuberculosis have been reported [4].With the global rise in Mycobacterium tuberculosis (Mtb) resistance rates, a more rapid and accurate susceptibility test is of primary importance to identify the resistant strains and initiate proper treatment in a timely manner[5, 6].Antimicrobial testing for Mycobacterium tuberculosis can be challenging to perform routinely in the clinical laboratory. The gold standard method for Mycobacterium tuberculosis complex susceptibility testing is the 1% indirect agar proportion method (APM). However, APM requires 2 to 3 weeks for results [7].The agar proportion method, while considered the "gold" standard of antibiotic susceptibility testing, is labor intensive and requires calculation of resistance by performing colony counts on drug-containing agar as compared to drug-free agar [8].The Trek Sensititre MYCOTB MIC plate (MYCOTB; Trek Diagnostic Systems, Cleveland, OH) is a dry microdilution plate. The plate uses a 96-well microtiter broth format containing lyophilized antibiotics, with concentrations prepared and quality controlled by the manufacturer. The MYCOTB plate is configured for determination of the minimal inhibitory concentrations (MICs) of first- and second-line anti-TB drugs [9]. In contrast to other M.tuberculosis complex susceptibility methods, which test one or two critical concentrations of a drug, the MYCOTB plate examines a range of drug concentrations and produces a MIC result [10].The objective of this study was to compare the Sensititre MTYCO TB MIC panel to the APM as regards accuracy, time to results and ease of use for both first and second line drugs.
2. Material and Methods
- This study was conducted in Mycobacteriology Laboratory-Ministry of Health, Central Labs in the period from January till July 2013. Forty clinical isolates including MDR and XDR strains were selected from archived isolates. These clinical isolates were from sputum specimens of tuberculosis patients.All isolates were subcultured on Lowenstein–Jensen media before testing was initiated. The first and second line drug susceptibilities were tested by both the Sensititre MYCOTB plate (TREK Diagnostics, Cleveland, OH) MIC commercial kit and the agar proportion method on LJ medium. The results of both methods were compared in terms of 12 anti-tuberculosis drugs (isoniazid, rifampin, ethambutol, ethionamide, cycloserine, kanamycin, streptomycin, amikacin, ofloxacin, rifabutin, moxifloxacin and para-aminosalicylic acid).
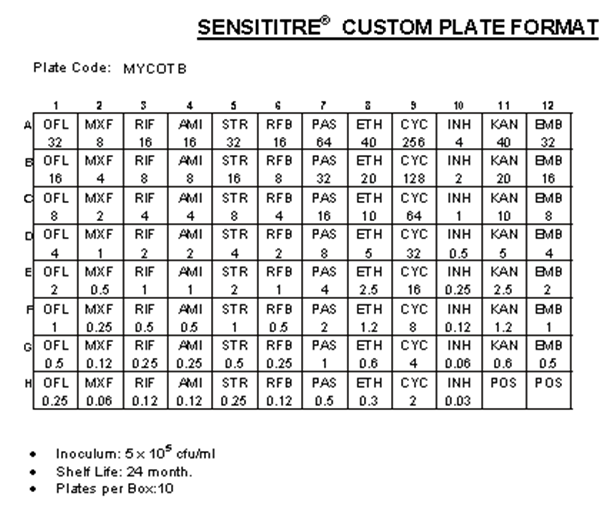
2.1. The Sensititre MYCOTB Method
- The whole procedure was performed according to the instructions of the manufacture (TREK Diagnostics, Cleveland, Ohio) (Figure 1). The Sensititre plate is a dry microdilution plate containing lyophilized antibiotics with different concentrations prepared and quality controlled by the manufacturer.
2.2. Gold Standard the Indirect Agar Proportion Method (APM)
- The APM was performed on LJ medium according to Canetti et al., 1969 [11] and Visalakshi et al., 2010 [12]. In brief, with a spatula, colonies were taken from the primary culture and placed in a spherical, flat-bottomed flask containing 30 glass beads 3 mm in diameter. The flask was shaken for 20-30 seconds; 5 ml of distilled water were added slowly under continuous shaking. The opacity of the bacterial suspension was then adjusted by the addition of distilled water to 1 McFarland standard. The inoculum for each slope is 0.1 ml. The two bacterial dilutions required for inoculation using automated pipettes were 10-3 mg/ml and 10-5 mg/ml. The dilutions were prepared by 10-fold dilution steps (0.5 ml of the bacterial suspension 1 mg/ml, discharged into 4.5 ml of distilled water, produces the dilution 10-1mg/ml; 0.5 ml of the bacterial suspension 10-1 mg/ml, discharged into 4.5 ml of distilled water, produced the dilution 10-2 mg/ml, etc., down to 10-5 mg/ml). Two slopes of medium without drug and 2 slopes of medium with drug were inoculated with 0.1 ml of the 2 chosen dilutions. The results were read for the first time on the 21st day. Interpretation was done according to Varma et al, 2002 [13]. The average number of colonies obtained for the 2 control slopes indicates the number of cultivable particles contained in the inoculum. The average number of colonies obtained for the drug-containing slopes indicates the number of resistant bacilli contained in the inoculum. The ratio between the second figure and the first indicates the proportion of resistant bacilli existing in the strain. Below a certain proportion (the critical proportion) the strain is classified as sensitive; and above, as resistant. Where growth of more than 1% of the inoculum on antibiotic- containing medium compared to inoculum on antibiotic- free control was taken to indicate resistance.Statistical analysis: Sensitivity, specificity of MYCOTB plate and categorical agreement with APM were calculated as followsSensitivity = (number of APM-resistant isolates identified as resistant by MYCOTB)/ [(number of APM-resistant isolates identified as resistant by MYCOTB)/ (number of APM-resistant isolates identified as susceptible by MYCOTB)].Specificity = (number of APM-susceptible isolates identified as susceptible by MYCOTB)/ [(number of APM-susceptible isolates identified as susceptible by MYCOTB) + (number of APM-susceptible isolates identified as resistant by MYCOTB)]. Categorical agreement between MYCOTB and APM: There was considered to be categorical agreement if both MYCOTB and APM characterized the isolate as susceptible or if both MYCOTB and APM characterized the isolate as resistant.
3. Results
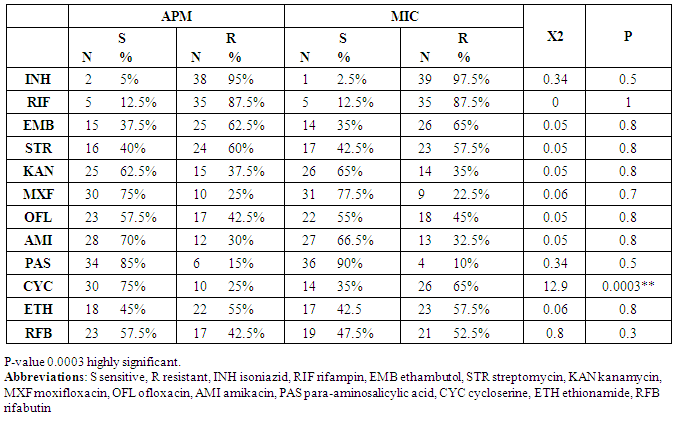
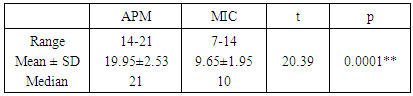
- The current study compared between APM and MYCOTB (MIC) as regard the number of Resistant isolates for each of the first and second line antituberculous drugs. A statistical significant difference between the results of the two methods was detected only for cycloserine, ten strains (25%) were resistant by APM versus 26 strains (65%) were resistant by MYCOTM (MIC) with p value = 0.0003 (table 1).
|
|
|
|
4. Discussion
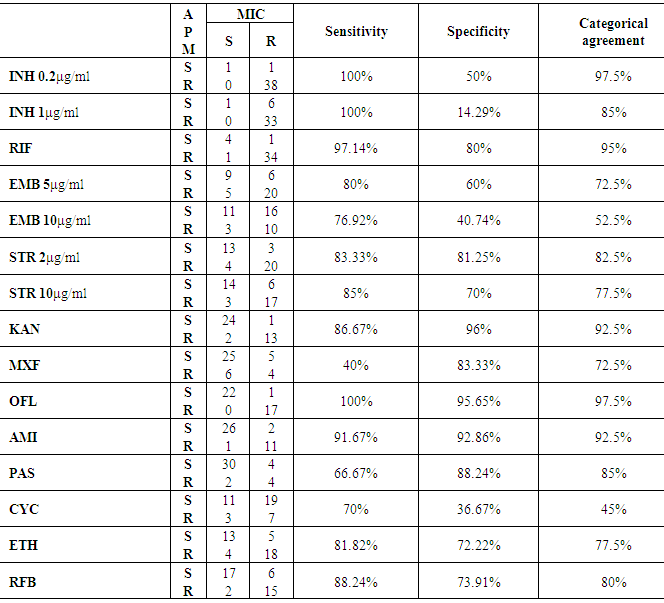
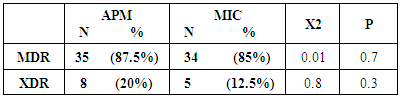
- Knowledge of M.tuberculosis drug susceptibility is important in optimizing individual patient management and TB control in populations. The reference phenotypic method is the indirect agar proportion (APM), which is qualitative and based on drug critical concentration. Limitations of this method include lack of standardization and in some cases the need of laboratory preparation of drug stokes and agar plates, which can be a source of variability over time and between laboratories [14].Critical concentrations are based on historical epidemiological data and for some drugs are not well aligned with achievable drug serum concentrations or accurate in predicting clinical failure [15]. There is an increased demand for reliable drug susceptibility testing (DST) [16].The current study assessed the performance and feasibility of the use of MYCOTB plate for drug susceptibility testing of 40 Mycobacterium Tuberculosis strains.No statistical significant difference was found between the results of Sensititre plate and APM regarding any of first or second line anti-tuberculosis drugs except with cycloserine P-value = 0.0003. The Sensititre plate detected higher number of resistant strains to cycloserine (26/40) than APM (10/40).The discrepancy of these results could be attributed to inactivation of the test drug during incubation of MIC plate [17]. Or difficulty in visualization of colonies of APM even with the aid of special light and magnifying glass [10].Lee et al, 2014 [9] detected discrepant results between APM and sensititer plate for moxifloxacin as large numbers of isolates that were categorized resistant by MYCOTB and susceptible by APM. DNA sequencing resolved the discrepancy in favor of the MYCOTB plate.The sensitivity of MYCOTB plate to detect TB sensitivity to the first and second classes of anti-tuberculosis drugs was determined in comparison to the APM as a gold standard. The highest sensitivity was to isoniazid 0.2μg/ml, 1μg/ml and ofloxacin 100%. This was in agreement with Lee et al, 2014 [9].The least sensitivity of Sensititer in the current study was to moxifloxacin 40%. The sensitivity to ethambutol 5μg/ml and cyclosirine were 80% and 70% respectively.Variable sensitivities were detected among different investigators; Lee et al, 2014 [9] found lower sensitivity to cycloserin (18.2%, versus 70% in the current study) and to ethambutol (31% versus 80% in concentration 5μg/ml and 76% in concentration 10μg/ml in the current study). On the other hand Hall et al, 2012 [18] detected higher sensitivity to ethambutol 10μg/ml than the current study (100%).As regard specificity of MYCOTB plate to detect TB resistance to first and second anti-tuberculosis. The highest specificity was to kanamycin, ofloxacin and amikacin 96%, 95.65% and 92.86% respectively. Our results were near to the results of Lee et al, 2014 [9] who found specificity to kanamycin, ofloxacin and amikacin was 98.8%, 91.8% and 100% respectively. The lowest specificity in the current study was to isoniazid 1μg/ml 14.29% and cyclosiren 36.67%. In contrast to our results Lee et al, 2014 [9] found that the least specificity was to moxifloxacin 0.5μg/ml 79.5% and all other tested drugs have high specificity between 81.3% to 100%.The categorical agreement between APM and Sensititer plate ranged from 45% to 97.5% in the current study. The highest agreement was detected for isonazid 0.2μg/ml and ofloxacin 97.5%. The least categorical agreement was with cycloserine 45%. Hall et al, 2012 [18] detected higher categorical agreement (94%-100%) comparing the Sensititre MYCOTB plate versus APM for first and second line anti tuberculosis drugs. They tested higher number of strains (122 isolates). Categorical agreement between the MIC by Sensititre Myco TB plate quantitative method and the qualitative APM was above 92% in 5 out of the 12 tested drugs (kanamycin, ofloxacin, amikacin, rifampin and isoniazid 0.2μg/ml). The categorical agreement between APM and Sensititer plate ranged between 52% and 82.5% for ethambutol, ethionamide and streptomycin. Abuali et al, 2011 [10] found that, the majority of Sensititre errors occurred with three drugs, ethambutol, ethionamide and streptomycin. Improving the sensitivity and specificity of susceptibility testing by enhancing the MYCOTB panel specifically for these drugs will need to be further explored. Abuali et al, 2011 [10] noted that historically streptomycin and ethambutol testing with the APM has yielded the most variable and inconsistent results. These errors might be encountered when comparing the MYCOTB panel value results with the APM resistant patterns of these drugs due to problem already existing of the APM and not actual errors.Ethambutol phenotypic DST is well known to be challenging with low interlaboratory agreement [19, 20].The current study didn’t detect any statistical significant difference between APM and Sensititer plate in detection of MDR-TB and XDR-TB.Salee et al, 2014 [21] noted that, by combining Sensititre MYCOTB test with the microscopic observation drug susceptibility (MODS) assay for detection of MDR and XDR-TB it was possible to identify MDR-TB as little as 3 days and XDR-TB within five days.We found that APM provides reliable results after incubation for 21 days, and before that time the growth was poor. On the other hand the Sensititre MYCOTB plate quantitative method in this study displayed consistent results by day 10.This was in agreement with Abuali et al, 2011 [10] who found that the procedure of the APM involves waiting 21 days for the susceptibility results of first line drugs, which are tested first prior to setting up second line drugs. They also noted that such a procedure results in significant delay of up to two months in attaining and reporting the susceptibility results to second line drugs. In conclusion: The MYCOTB plate does not need instrumentation for interpreting the results and yielded results in shorter time than APM with advantage of including second line anti-tuberculosis drugs in the same plate. Concordance was high between APM and the MYCOTB plate except for cycloserin and ethambutol in concentration 10μg/ml. Sequencing is needed to identify the cause of discrepant results in these two antibiotics.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML