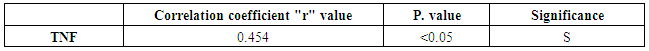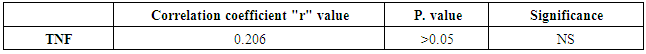Radwa S. Shahin1, Sahar Saad Zalam1, Wael Mohamed Saudi2, Amany M. Abdallah3
1Clinical Pathology Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Dermatology and Venereology, Faculty of Medicine, Miser University for science and technology, Egypt
3Internal Medicine Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Radwa S. Shahin, Clinical Pathology Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: CD26 is a multifunctional type II transmembrane glycoprotein, which also exists as a secreted isoform, soluble CD26 (sCD26). The CD26 expression on circulating T cells is decreased in some skin diseases such as psoriasis. It remains to be determined whether sCD26 can be used as a marker of skin diseases or not.Objective To investigate the utility of sCD26 as a diagnostic marker of psoriasis (Ps) and/ or psoriatic arthritis (PsA) in combination with TNF-α.Methods: Serum sCD26 and TNF-α Levels were measured using enzyme-linked immunosorbent assay in 36 patients, including 18 patients with psoriasis; 18 patients with psoriatic arthritis and 18 healthy controls.Results: Our results show a highly significant decrease in mean CD26 levels in Ps and PsA when compared tocontrol group and when the groups compared to each other, where there was a significant increase in mean TNF levels in Ps and PsA as compared tocontrol group and when the groups compared to each other. There was a significant positive correlation between CD26 and TNF and between PASI score and TNF, while there is no significant correlation between PASI score and CD26 in PS. There was no significant correlation between CD26 and TNF and between PASI score and CD26 and TNF in PSA. Conclusions: Serum sCD26 levels, combined with serum TNF-α Level are helpful in diagnosis of psoriasis and psoriatic arthritis.
Keywords:
CD26, TNF-α., Psoriasis (Ps) and psoriatic arthritis (PsA)
Cite this paper: Radwa S. Shahin, Sahar Saad Zalam, Wael Mohamed Saudi, Amany M. Abdallah, Assessment of Serum CD26 Levels in Psoriasis and Psoriatic Arthritis in Combination with TNF-α Levels and Their Relation to Disease Severity, Clinical Medicine and Diagnostics, Vol. 5 No. 5, 2015, pp. 97-106. doi: 10.5923/j.cmd.20150505.03.
1. Introduction
Psoriasis (Ps) is a common, chronic, inflammatory, immune-mediated, multisystem disease that has predominantly skin and joint manifestations results from a polygenic predisposition combined with triggering factors, e.g. trauma, infections or medications [11].Psoriatic arthritis (PsA) is an inflammatory arthritis, which is typically associated with psoriasis and psoriatic nail disease. It has both peripheral articular manifestations (including synovitis, dactylitis, and enthesitis) and axial skeletal involvement. A range of bone pathologies was observed in patients with PsA including aberrant bone loss and new bone formation [19]. Now, It is apparent that PsA is more aggressive than previously thought and the majority of patients with PsA experience a chronic, progressive course. Approximately one-fifth of patients with PsA develop into a destructive, disabling form of arthritis over time. Two main cell types are involved in bone remodeling: osteoclasts and osteoblasts [2].CD26, exhibiting dipeptidyl peptidase IV enzyme activity, is a multifunctional type II transmembrane glycoprotein, which belongs to a serine protease family. It is expressed in a variety of tissues, including epithelial cells, endothelial cells, natural killer cells and the subset of T cells. It is up-regulated after T cells and natural killer cells get activated. CD26 acts to selectively remove the N-terminal dipeptide from peptides with proline or alanin in the penultimate position [12].Several gastrointestinal hormones, neuropeptides, integrins and chemokines have been demonstrated to be cleaved by this enzymatic activity. CD26 also exhibits a co-stimulatory function. It can bind adenosine deaminase and mediate signalling by direct interaction with the cytoplasmic domain of CD45. A new model for CD26 costimulatory function suggests that, at least in activated memory T cells, CD26 enhances antigen-specific T cell proliferation by engaging signaling pathways in the antigen presenting cells, in particular, the up-regulation of CD86 [3].There are many studies reported on CD26 involvement in skin diseases. Expression of CD26 on CD8+ T cells is decreased in patients with psoriasis. Increased expression of CD26 on keratinocytes is observed in CTCL and inflammatory skin diseases as psoriasis. CD26 also exists as a secreted isoform, soluble CD26 (sCD26), which lacks the cytoplasmic tail and transmembrane region. It is reported to have the same function or enzymatic activity as the membrane form of CD26 [12].In this study, we measured serum sCD26 and TNF-α levels in patients with psoriasis and Psoriatic arthritis aiming to investigate their utility as a diagnostic markers and to assess their relationship with disease severity.
2. Subjects and Methods
This study was conducted on 36 patients suffering from psoriasis, 18 of them psoriasis without arthritis (PS group) and 18 were psoriatic arthritis (PSA group). Patients were selected from the Dermatology Out patients Clinic of Dermatology and Venereology of Al-Zahraa University Hospital and Dermatology and Venereology Department of Miser University for science and technology.Eighteen apparently healthy persons from the hospital workers with no history of skin or autoimmune diseases were selected to serve as control matching on age and sex. Full consent was taken from all the participants after the approval of the ethics committee of the faculty of medicine for girls, Al-Azhar University. *Criteria for selection:Cases were selected according to the following criteria.• Inclusion criteria:- 1- Both male and female aged >18 years old.2- Any type of psoriasis was included.3- Diagnosis of psoriatic arthritis was done according Classification Criteria for Psoriatic Arthritis (CASPAR) criteria. [15]• Exclusion criteria:a. Intake of systemic antipsoriatic drugs including methotrexate-cyclosporine–biological agent 4 weeks before the study and six months for retinoids.b. Thyroid dysfunction.c. Renal and Liver diseases.d. Diabetes mellitus and Cardiovascular diseases.e. Malignancies.f. Presence of autoimmune diseases, infections or endocrinal diseasesg. Presence of joint diseases in psoratic patients.All patients were subjected to:1 – Full medical history:− Personal history, including name, age, sex, marital status, address, occupation, telephone number and special habits of medical importance.− History of present illness, including onset, course, duration of the disease, previous treatment and the date of stoppage of the last treatment modality.− Family history of psoriasis.− History of other skin or systemic diseases.2 – Full medical examination: - Careful general examination: including joint examination and search for clinical manifestations suggestive of any systemic disease.- Dermatological examination: the degree of psoriasis' severity was scored by Psoriasis Area Severity Index (PASI) score and body surface area (BSA) involved in the disease as well as examination of the nails and scalps.- Calculation of Psoriasis Area Severity Index (PASI) score:Four sites of affection, the head (H), upper limb (U), trunk (T) and lower limbs (L), are separately scored by using three parameters, erythema, induration and desquamation, each of which was graded on a severity scale of 0 to 4, where 0 = nil, 1 = mild, 2 = moderate, 3 = severe and 4 = very severe. The surface area involved (per body region) was calculated as: 1 = less than 10% area; 2 = 10-29%; 3 = 30-49%; 4 = 50-69%; 5 = 70-89%; and 6 = more than 90%. The body surface area was calculated as: H=10%; U=20%; T=30%; L=40%The final formula for PASI score is:PASI = 0.1 (EH + IH + DH) AH + 0.2 (EU + IU + DU) AU + 0.3 (ET + IT+ DT) AT + 0.4 (EL + IL + DL) ALThe maximum score of PASI is 72 [6]According to PASI score:-− Mild psoriasis: 20 or less.− Moderate psoriasis: ranged from 21 to 50.− Severe psoriasis: more than 50.− Erythrodermic, pustular and palmoplantar psoriasis are considered severe psoriasis [7].3 - Joint examination: It includes assessment of swelling, tenderness, warmth (hotness), crepitation, range of motion.4 - Plain X-ray:- X-ray was done on the hands and feet and/ or affected joint of all psoriatic arthritis patients.Radiographic features of PsA are the result of a combination of erosive and proliferative bone changes. Characteristic findings include: [7]- Enthesitis and marginal bone erosions; "pencil-in-cup" deformities are common, but not pathognomonic for PsA - Joint subluxation or interphalangeal ankylosis may be present.- Bone proliferation results in an irregular, “fuzzy” appearance of the bone around the affected joint - Periostitis: may appear as a periosteal layer of new bone, or as irregular thickening of the cortex itself - Dactylitis: which can present as a “sausage digit” which refers to soft tissue swelling of a whole digit; ultrasound examination of a sausage digit demonstrates underlying synovitis and tenosynovitis - Arthritis mutilans: a severe form of either PsA or rheumatoid arthritis caused by marked bony resorption and the consequent collapse of soft tissue; when this affects the hands, it can cause a phenomenon sometimes referred to as "telescoping fingers"- Ivory phalanx: classically involving the distal phalanx of the great toe- Sacroiliitis: often asymmetrical- Spondylitis: asymmetric paravertebral ossifications and relative sparing of the facet joints 4 - Lab investigations: Blood samples were taken from patients and control subjects. Each blood sample was put in a plan tube, left to clot, then centrifuged at 1000 rpm for 15 minutes and serum was separated and used for estimation of:− Assessment of serum CD26: was detected by ELISA immunoassay Kits from R&D Systems, Minneapolis, MN 55413, USA. Catalogue number DC260. Briefly, standards, samples and controls were pipetted into wells, pre-coated with A polyclonal antibody specific for the DPPIV (sCD26) and any the DPPIV (sCD26) present is bound by the immobilized antibody. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for the DPPIV (sCD26) is added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of sCD26 bound in the initial step. The color development is stopped and the intensity of the color is measured and the microtiter plate was then read at 450nm wave length. The level of sCD26 was calculated from the standard curve corresponding to the measured optical density. The results were expressed as ng/ml. Minimum detectable limit of sCD26 ranged from 0. -ng/ml.− Assessment of serum TNF-α: performed by Enzyme Linked Immuno Sorbent Assay (ELISA). The kit assays Human TNF-α level in the sample, use Purified antibody specific to TNF-α to coat microtiter plate wells, make solid-phase antibody, standards or samples are then added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for TNF-α and avidin conjugated to horseradish peroxidase (HRP) is added to each microplate well and incubated. Then add TMB solution, only those wells that contain TNF-α, biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit changes in color. The enzyme-substrate reaction is terminated by the addition of a sulfuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450 nm ± 2nm.The concentration of TNF-α in the samples is then determined by comparing the O.D. of the samples to the standard curve.- Statistical analysis: Data were analyzed using SPSS (Statistical Package For Social Science) version 17.0 and Microsoft Excel 2010. Parametric data were expressed as mean ± SD and non-parametric data were expressed as number and percentage. Student’s t test was done to compare between two groups. Analysis of Variance (ANOVA) test, was used to estimate the difference between the means of more than two groups. Chi-square test: was used to examine the relationship between two qualitative variables. Pearson Correlation Coefficient was done to correlate between different parameters among groups. The p value of > 0.05 considered non significant, p value of ≤ 0.05 considered significant, p value of < 0.01 was considered highly significant.
3. Results
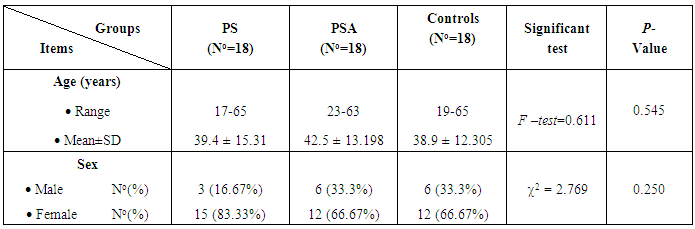
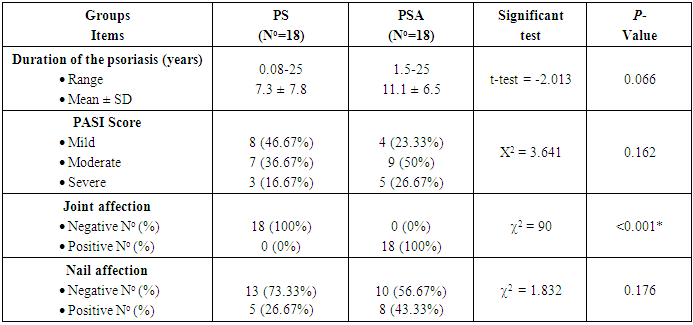
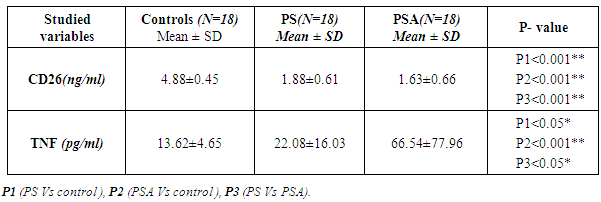
The results and data were collected and analyzed in tables 1-7 and figures 1-8- Comparison between the studied groups regarding demographic data Table (1): - In PS group: duration of psoriasis ranged from 0.08 -25 years (mean ± SD=7.3 ±7.8). No patients had joint affection, 5 patients had nail affection. In PSA group: duration of psoriasis ranged from 1.5-25 years (mean ± SD = 11.05±6.54), all patients had joint affection and 8 patients had nail affection. There was no statistical significant difference between psoriatic patient without arthritis and psoriatic arthritis as regards demographic data.- Comparison between psoriatic patients and psoriatic arthritis patients regarding clinical data (Table 2): There was a statistical significant difference between Ps and PSA groups as regards duration of psoriasis, while, there was no statistically significant difference regarding nail affection.- Comparison between mean CD26 and TNF levels among all groups Table (3): There was a highly significant decrease in mean CD26 levels in PS and PSA groups as compared to control group and when the groups compared to each other's. There was a significant increase in mean TNF levels in PS and PSA groups as compared to control group and when the groups compared to each other- Correlation between CD26 and TNF in PS group Table (4): There was a significant positive correlation between CD26 and TNF in PS group.- Correlation between PASI score and other parameters in PS group Table (5):. There was a significant positive correlation between PASI score and TNF, while there is no significant correlation between PASI score and CD26 in PS group. - Correlation between CD26 and TNF in PSA group Table (6): There is no significant correlation between CD26 and TNF in PSA. - Correlation between PASI and other parameters in PSA group Table (7): There was no significant correlation between PASI score and CD26 and TNF in PSA group. Table (1). Comparison between the studied groups regarding demographic data
 |
| |
|
Table (2). Comparison between psoriatic patients and psoriatic arthritis patients regarding clinical data
 |
| |
|
Table (3). Comparison between mean CD26 and TNF levels among all groups
 |
| |
|
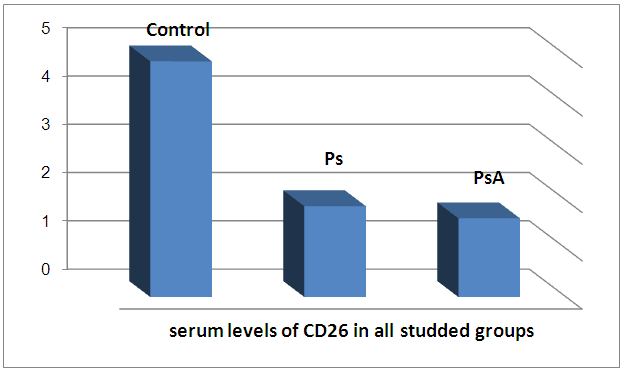 | Figure 1. Serum levels of CD26 in all studied groups |
There is a highly significant decrease in mean CD26 levels in PS and PSA groups as compared to control group and when the groups compared to each other. There is a significant increase in mean TNF levels in PS and PSA groups as compared to control group and when the groups compared to each other.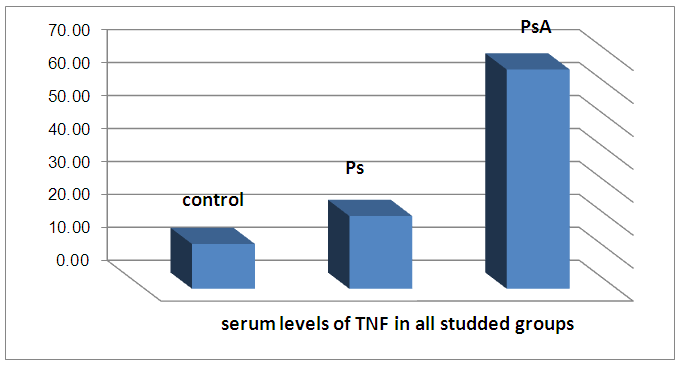 | Figure 2. Serum levels of TNF in all groups |
Table (4). Correlation between CD26 and TNF in PS group
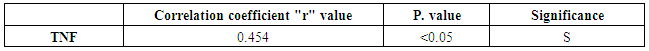 |
| |
|
There was a significant positive correlation between CD26 and TNF in PS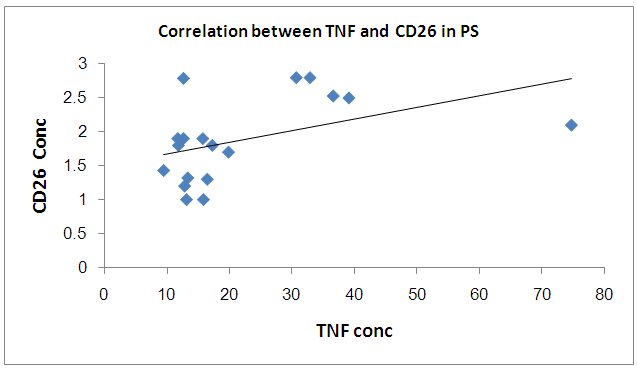 | Figure 3. Correlation between TNF and CD26 in PS group |
Table (5). Correlation between PASI score and other parameters in PS group
 |
| |
|
There was significant positive correlation between PASI score and TNF while there is no significant correlation between PASI score and CD26 in PS group 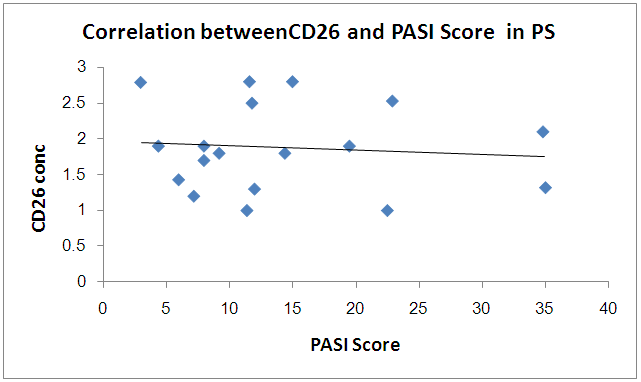 | Figure 4. Correlation betweenCD26 and PASI Score in PS group |
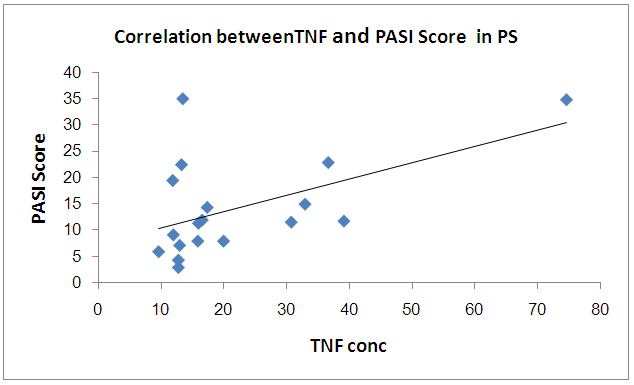 | Figure 5. Correlation between TNF and PASI Score in PS group |
Table (6). Correlation between CD26 and TNF in PSA group
 |
| |
|
There is no significant correlation between CD26 and TNF in PSA group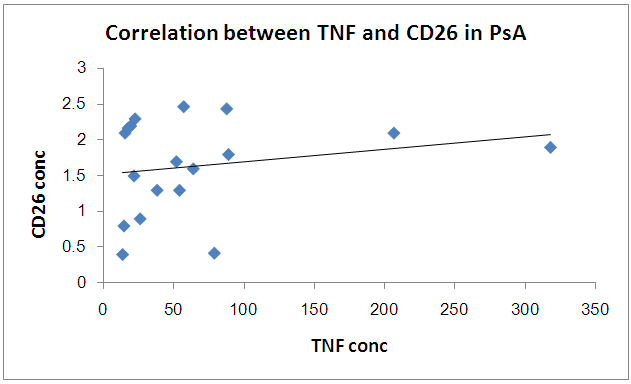 | Figure 6. Correlation between TNF and CD26 in PSA group |
Table (7). Correlation between PASI and other parameters in PSA group
 |
| |
|
There is no significant correlation between PASI score and CD26 and TNF in PSA group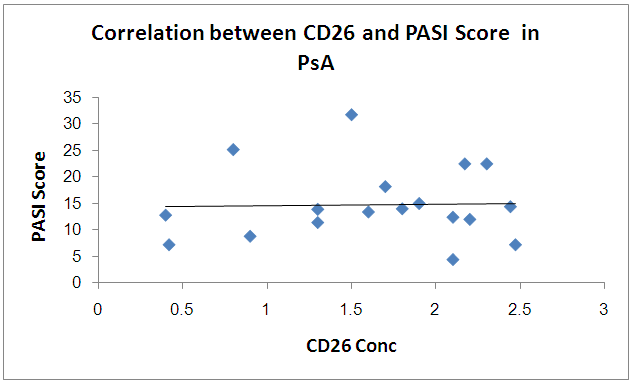 | Figure 7. Correlation betweenCD26 and PASI Score in PSA group |
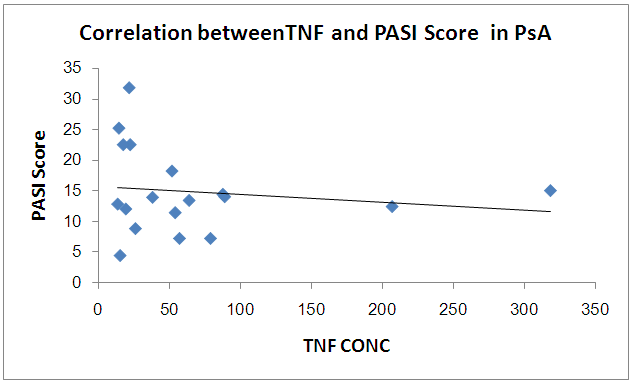 | Figure 8. Correlation between TNF and PASI Score in PSA group |
4. Discussion
The prevalence of psoriasis is approximately 1–5%. Although the etiology of psoriasis is not completely understood, it is thought to be an autoimmune, inflammatory disease characterized by the activation of keratinocytes secondary to lymphocyte activation in the dermis and epidermis. Although the sequence of events between keratinocyte and immune-cell activation in psoriasis remains unclear, it has been shown that T cells are important in immunopathogenesis. Activated T lymphocytes secrete cytokines, including interleukin (IL)-1, IL-2, IL-6, IL-8, tumor necrosis factor (TNF)-α, interferon γ, epidermal growth factor, fibroblast growth factor and platelet-mediated growth factor [1].Psoriatic arthritis (PsA) is a chronic inflammatory skin disease that causes enthesitis and destructive arthritis and significantly lowers patient quality of life. Recognition of the two target organs (the skin and joints) involved in the immunopathophysiology of PsA helped in elucidating the pathology of various systemic autoimmune diseases targeting multiple organs. Advances in immunology and genetics have made it clear that acquired immunity, especially that mediated by the Th17/IL-23 axis, plays an important role in the inflammatory pathology observed in psoriasis and PsA [9].CD26/dipeptidylpeptidase IV (DPPIV) is a multifunctional glycoprotein known to be present and upregulated on the surface of keratinocytes and T cells in psoriatic skin compared with the skin of healthy volunteers. The expression of CD26/DPPIV on peripheral blood T-lymphocytes of untreated psoriatic patients, however, was found to be substantially reduced, in particular on the CD8+CD26bright subset. Furthermore, TNF-α has been reported to influence CD26/DPPIV expression. In vitro cell cultures have shown that anti-TNF-α can upregulate the expression of CD26/DPPIV on human-activated lymphocytes [16]. In this study, we measured the serum levels of CD26 in PS and PsA patients and the authors found that there was a highly significant decrease in mean CD26 levels in Ps and PsA patients as compared to control group and when the groups compared to each other.This results were in agreement of Miyagaki et al, 2013 [12] whom reported that CD26 expression in peripheral blood T cells is reduced in patients with psoriasis. Serum sCD26 is thought to be shed from plasma membrane on CD26 expressing cells that are in contact with blood, such as circulating lymphocytes and vascular endothelial cells. In healthy individuals, the electrophoretic mobility of normal serum sCD26 coincides with that of membranous CD26 on lymphocyte, but not with CD26 purified from liver or kidney, abundant with vascular endothelial cells. Moreover, immune reconstitution after anti-retroviral therapy not only increases serum sCD26 activity, but also the number of CD26+ T lymphocytes. Based on these reports, the main source of serum sCD26 should be circulating CD26+ lymphocytes. The authors also found that there was a significant increase in mean TNFα levels in Ps and PsA as compared to control group and when the groups compared to each other. These results were in accordance with Xue, et al 2012 [19], whom found that Elevated concentrations of the proinflammatory cytokine, tumor necrosis factor (TNF-α), have been detected in the joint synovium and lesional skin of patients with PsA. Subsequently, TNF has been validated as a therapeutic target in PsA and several other immune-mediated inflammatory diseases. Anti-TNF-a biologic therapies have been demonstrated to significantly reduce the signs and symptoms of PsA. Their data showed that the circulating concentrations of TNF-a in patients with PsA were higher than those in both healthy controls and psoriasis controls.Our results were also in agreement of Ragab, et al 2010 [15], who found that Serum TNF-α, IL-2R and IL-6 were all statistically significantly elevated in the psoriasis group as compared to healthy controls. Our results also are in agreement with earlier studies demonstrated by Mohammad 2005 [13], who showed a significant increase in levels of serum IL-6, IL-8, IL-2R and TNF-α in Saudi psoriasis patients as compared with healthy controls. And he has been suggested that proinflammatory cytokines not only play a fundamental role in the worsening of the disease or activating its pathogenetic mechanisms, but are also directly related to the clinical symptoms and disease evolution after effective therapy. Also, similar to our results, Ozer et al. 2005 [14] have demonstrated that, serum TNF-[alpha], IFN-[gamma], IL-6, IL-8, IL-12, and IL-18 levels were significantly higher in active psoriatic patients than in controls.In Japanese patients with psoriasis, Takahashi et al. 2009 [17] have shown that serum levels of tumor necrosis factor (TNF)-alpha, interferon (IFN)-gamma, interleukin IL-2, IL-6, IL-7, IL-8, IL-12, IL-17, IL-18 and vascular endothelial growth factor (VEGF) were significantly increased in patients with psoriasis compared with those of healthy controls.Our results show there were significant positive correlation between CD26 and TNF-α in PS while There is no significant correlation between CD26 and TNF-α in PSA.As regards to the correlation between disease severity (PASI) and CD26, TNF-α our results show there ware significant positive correlation between PASI and TNF-α while there is no significant correlation between PASI score and CD26 in PS. The Dilek et al 2013 study [5] show a correct relationship between PASI scores and TNF-α.Takahashi et al. 2009 [17] have shown that serum levels of tumor necrosis factor (TNF)-alpha, and other cytokines were correlated with PASI. Furthermore, these cytokine levels were decreased after psoriasis treatment.Similar to our results, Ozer et al. 2005 [14] have demonstrated that, serum TNF - [alpha], IFN - [gamma], IL - 6, IL - 8, IL - 12, and IL - 18 levels were significantly higher in active psoriatic patients than in controls. Furthermore, high levels of these parameters have been correlated with the clinical severity and activity of psoriasis, and they concluded that measurements of serum levels of these cytokines may be objective parameters for the disease severity.Our results not in agreement with the results obtained by Deeva et al. 2010 [4], whom found no significant correlation between psoriasis severity assessed by PASI (Psoriasis Area and Severity Index) and levels of (TNF)-alpha, and other cytokines.Rosanne, et al, 2009 [16] reported that CD26 expression may have a crucial role and might function as a marker correlating with disease activity in the pathogenesis of psoriasis, which against our results showing significant positive correlation between PASI and TNF-α but not with CD26. There is no significant correlation between PASI and CD26 and TNFα in PSA patients. Our results were in agreement of Mastroiann, et al 2005 [10] who found that PASI was not correlated with TNF-alpha in the serum of PsA patients.
5. Conclusions
- We found that sCD26 levels are significantly lower in patients with psoriasis and psoriatic arthritis. On the other hand, serum level of TNF-α are significantly elevated in patients suffering from Ps and PsA. With increase of the severity of the disease, TNF-α is significantly elevated in PsA patients than in Ps which is attributed to the role of these cytokines in the pathogenesis and progression of psoriasis and its elevation is responsible for the development, maintenance and resolution of psoriatic lesion.
References
| [1] | Akçali1 Cenk, Guven Ebru Homurlu, Kirtak Necmettin , H Serhat Inaloz, Orhan Ozgoztasi, Ulas Guvenc. (2014): Serum concentrations of interleukin-2 and tumour necrosis factor-α under cyclosporine versus acitretin treatment in plaque-type psoriasis.Journal of International Medical Research October 2014 vol. 42 no. 5 1118-1122. |
| [2] | Anandarajah AP, Schwarz EM, Totterman S, Monu J, Feng CY, et al. (2008) The effect of etanercept on osteoclast precursor frequency and enhancing bone marrow oedema in patients with psoriatic arthritis. Ann Rheum Dis 67:296–301. |
| [3] | Cordero OJ, Salgado FJ, Nogueira M (2009). On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol Immunother; 58: 1723–1747. |
| [4] | Deeva I., Mariani S., De Luca C., Pacifico V., Leoni L., Raskovic D., Kharaeva Z., Korkina L., Pastore S (2010). Wide-spectrum profile of inflammatory mediators in the plasma and scales of patients with psoriatic disease. Cytokine. 49(2):163-70. |
| [5] | Dilek AR1, Dilek N, Saral Y, Yüksel D (2013). The relationship between severity of the disease and circulating nucleosomes in psoriasis patients. Arch Dermatol Res. Aug; 305(6): 483-7. |
| [6] | Fredriksson T and Pettersson U (1978). Severe psoriasis--oral therapy with a new retinoid. In Dermatologica. 157: 238-44. |
| [7] | Jacobson JA, Girish G, Jiang Y (2008). Radiographic evaluation of arthritis: inflammatory conditions. Radiology.; 248 (2): 378-89. |
| [8] | Louden BA, Pearce DJ, Lang W, Feldman SR (2004). A Simplified Psoriasis Area Severity Index (SPASI) for rating psoriasis severity in clinic patients. in Dermatol Online J.10: 7. |
| [9] | Maeda Shinji, Hayami Yoshihito, Naniwa Taio, and Ueda Ryuzo (2012): The Th17/IL-23 Axis and Natural Immunity in Psoriatic Arthritis. International Journal of Rheumatology Volume (2012), Article ID 539683, 8 pages. |
| [10] | Mastroianni A1, Minutilli E, Mussi A, Bordignon V, Trento E, D'Agosto G, Cordiali-Fei P, Berardesca E (2005). Cytokine profiles during infliximab monotherapy in psoriatic arthritis.Br J Dermatol. Sep; 153(3):531-6. |
| [11] | Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Leonardi CL, Lim HW, Van Voorhees AS, Beutner KB, Ryan C and Bhushan R (2011): Guidelines of care for the management of psoriasis and psoriatic arthritis Section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: Case-based presentations and evidence-based conclusions. J Am Acad Dermatol; 65: 137-74. |
| [12] | Miyagaki T., Sugaya M., Suga H., Morimura S., Kamata M., Ohmatsu H., Fujita H., Asano Y., Y. Tada Y., (2013): Serum soluble CD26 levels: diagnostic efficiency for atopic dermatitis, cutaneous T-cell lymphoma and psoriasis in combination with serum thymus and activation-regulated chemokine levels, JEADV 2013, 27, 19–24. |
| [13] | Mohammad T (2005). Serum levels of proinflammatory cytokines in psoriasis patients from Saudi Arabia. International Journal of Dermatology 2005; 44, 81–82. |
| [14] | Ozer A., Murat A., Sezai S. and Pinar C (2005). Serum levels of TNF-[alpha], IFN-[gamma], IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. 2005Mediators of Inflammation.; 5, 273–279. |
| [15] | Ragab Halla M., Abd El Maksoud Nabila and Roaiah Mohamed M. Farid (2010): Biochemical Significance of Proinflammatory Cytokines in Psoriasisvulgaris among Egyptian Patients. New York Science Journal 2010; 3(10)58-66. |
| [16] | Rosanne G. van Lingen, Piet E. J. van Erp, Marieke M. B. Seyger, Elke M. G. J. de Jong, Roelie T. de Boer-van Huizen, RiekeJ. B. Driessen and Peter C. M. van de Kerkhof (2009): Persistent Expression of CD26/DPPIV After Treatment with Infliximab in Psoriasis Despite Clinical Improvement. Acta Derm Venereol 89. |
| [17] | Takahashi H., Tsuji H., Hashimoto Y., Ishida- Yamamoto A., Iizuka H (2009). Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol. 2009; 19. |
| [18] | Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group (2006). Classification criteria for psoriatic arthritis: development of new criteria from a large international study.Arthritis Rheum; 54: 2665-73. |
| [19] | Xue Y, Jiang L, Cheng Q, Chen H, Yu Y, et al. (2012): Adipokines in Psoriatic Arthritis Patients: The Correlations with Osteoclast Precursors and BoneErosions. PLoS ONE 7(10): e46740. |











 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML