-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2015; 5(4): 53-59
doi:10.5923/j.cmd.20150504.01
Cord Blood Matrix Metalloproteinase - 9 as Predictor of Respiratory Outcome in Preterm Infants
Soma Abdalla Mohamad1, Heba S. Gafar1, Marwa Elhady1, Aida Ahmed Abdel Hameed2
1Department of pediatrics, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Clinical pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Marwa Elhady, Department of pediatrics, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Background:Preterm birth is an emerging global public health problem. Respiratory distress syndrome (RDS) is a main contributor to increased mortality and morbidity among premature infants. Matrix metalloproteinase dysregulation contributes to the pathology of chronic and acute lung disorders, including bronchopulmonary dysplasia, RDS of newborn, asthma and emphysema. AIM: To evaluate the value of cord blood matrix metalloproteinase-9 for prediction of respiratory outcome in preterm neonates. METHODS: Sixty nine preterm neonates are included in this study; 40 of them had developed RDS while 29 did not developed RDS. Cord blood level of MMP-9 was estimated. We investigated the correlation between MMP-9, gestational age and severity of RDS in preterm neonates. RESULTS: Levels of cord blood MMP-9 were 2.92 ± 2.315 ng/ml & 6.12 ± 4.14 ng/ml in patients with and without RDS respectively. MMP-9 was significantly lower in patients developed RDS than those without RDS (p<0.001). MMP-9 level had positive correlation with gestational age. CONCLUSIONS: Cord blood MMP-9 is a promising novel biomarker which could reflect lung immaturity and identify the severity of RDS in preterm neonates.
Keywords: RDS, Preterm, Cord blood, MMP-9
Cite this paper: Soma Abdalla Mohamad, Heba S. Gafar, Marwa Elhady, Aida Ahmed Abdel Hameed, Cord Blood Matrix Metalloproteinase - 9 as Predictor of Respiratory Outcome in Preterm Infants, Clinical Medicine and Diagnostics, Vol. 5 No. 4, 2015, pp. 53-59. doi: 10.5923/j.cmd.20150504.01.
1. Introduction
- Respiratory distress syndrome remains the most common problem in preterm neonates resulting in significant morbidity, mortality, and extra health care costs [1, 2]. Early identification of preterm neonates susceptible to RDS is important since they may benefit from prophylactic surfactant administration and/or less vigorous ventilation [3].Normal lung growth is controlled at a number of different levels. Epithelium–mesenchyme interactions are one of these levels that coordinate and adjust lung development by modulating the proliferation, differentiation, and apoptosis of lung tissue cells enabling formation of trachea–bronchial tree and alveoli [4].Metalloproteinases (MMPs) are a family of potent zinc-dependent enzymes [5]. MMP have the ability to degrade most of the stromal components, so they have a key role in degradation of intracellular space, as well as in remodeling of lung interstitium, epithelial basal membrane and blood vessel endothelium [6]. MMP also degrade cell receptors influencing proliferation and apoptosis [7]. The regulation of MMP activity is necessary for normal matrix turnover and contributes to the development of normal lung architecture [8]. MMP dysregulation contributes to the pathology of chronic and acute lung disorders [9]. In the last 10 years, research on MMP-9 has increased exponentially; making this enzyme the prototype of the MMP family and one of the most studied enzymes. MMP-9 is involved in fundamental biological processes including development, angiogenesis, apoptosis, inflammation and cancer [10]. MMP-9 is also involved in feto-neonatal development and it has a key role in development of lung injury and fibrosis [11]. Adequate lung development is the major factor that decisively affects the postnatal outcome in the perinatal period. Although previous studies demonstrated an association between increased levels of MMP-9 and bronchopulmonary dysplasia (BPD) [12], there is little information directly addressing the role of MMP-9 for development of RDS in preterm neonates. Our study aims to evaluate the value of cord blood MMP-9 for prediction of respiratory outcome in preterm neonates.
2. Patients and Methods
- This cross-sectional comparative study was carried out at NICU of AL-Zahraa hospital, Al-Azhar University Cairo, Egypt during the period from June 2014 to January 2015. Cord blood samples from a total of 69 preterm laboredpregnant women were collected at the time of the delivery. They were selected from the Department of Obstetrics and Gynaecology of AL-Zahraa hospital. Forty preterm infants who developed RDS served as case group and 29 preterm infants without RDS as controls. Informed consent was obtained from the participating parents in adherence with the guidelines of the ethical committee of AL-Zahraa hospital, AL-Azhar University, Cairo, Egypt.Eligible cases were defined as singleton live born preterm neonates < 36 6/7 weeks’ gestation. Neonates with genetic syndromes, congenital malformations, PROM >18 hours, and other associated pathologies (sepsis, asphyxia) were excluded.Clinical and demographic informationMaternal interview was conducted using a structured questionnaire that included demographic characteristics, medical and reproductive history. Medical record review was conducted using a standardized abstraction form that included data on prenatal care, pregnancy complications, antenatal steroid and birth outcomes. In addition to, natal history includes mode and duration of delivery. In all cases, information related to gestational age at birth, sex and birth weight were recorded. Maternal CRP was negative for all included infants.Determination of GAGA was assessed with an algorithm based on last menstrual period and early ultrasound before 20 weeks gestation [13], in addition to post natal assessment using New Ballard Score [14].Identification of RDSAll patients were subjected to a full general and local examination. Neonates were followed until discharge from the hospital, at which time relevant data from the nursery records was obtained. None of our patients received early rescue surfactant therapy. Chest X ray were performed within the 1st hour of life before administration of surfactant to detect radiological findings of RDS and assess its severity. RDS was diagnosed on a combination of clinical and radiographic features. Clinical signs of RDS include tachypnea (RR>60), retraction, grunting and cyanosis presenting within the first 4–6 h after birth [15-17]. In addition to typical radiographic findings on the chest X-ray that ranged from a light reticulogranular appearance with air bronchogram to white lung [18, 19]. Chest radiographs were classified into four grades according to Bomsel's classification [20, 21].Cord blood biomarker assaysThree ml cord blood without anticoagulant was withdrawn from venous cord blood under aseptic condition. Blood was centrifuged and serum was removed for assays of biochemical and inflammatory markers. All samples were allowed to clot for 2 hours at room temperature then centrifuged for 15 minutes at 1000xg. The sample should be clear and be centrifuged to remove solids. The supernatant was collected and stored at -80 until assays of serum Matrex Metalloproteinase 9 (MMP-9).MMP-9 level was assayed using ELISA Human MMP-9 Matrix Metalloproteinase ELISA KIT were obtained from (Elabscience) E-EL-H145.This ELISA KIT uses Sandwich ELISA as the method.The micro ELISA plate provided in this kit has been pre-coated with an antibody specific to MMP-9. Standards or samples are added to the appropriate micro ELISA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for MMP-9 and Avidin-Horseradish peroxidase (HRP) conjugate is added to each micro plate well successively and incubated free components are washed away. The substrate solution is added to each well. Only those wells that contain MMP-9, biotinylated detection antibody and Avidin-Hrp conjugate will appear blue in color. The enzyme substrate reaction is terminated by the addition of a sulphoric acid solution and the colour turns yellow. The optical density (OD) is measuredspectrophotometrically at a wave length 450nm-+2nm. The OD value is proportional to the concentration of MMP-9.Statistical analysisStatistical analysis was performed using the Statistical Package for Social Sciences (version 17.0; SPSS Inc., Chicago, IL, USA). Differences between groups were analyzed with the Mann‑Whitney U test, paired t‑test and ANOVA test. Non paragramatic data were compared by chi square test. Correlations between groups were performed using Spearman correlation coefficients. Receiver operating characteristic curves (ROC) were used to identify the optimal cut-off points of MMP-9 level for prediction of RDS. P-value < 0.05 was considered to be significant.
3. Results
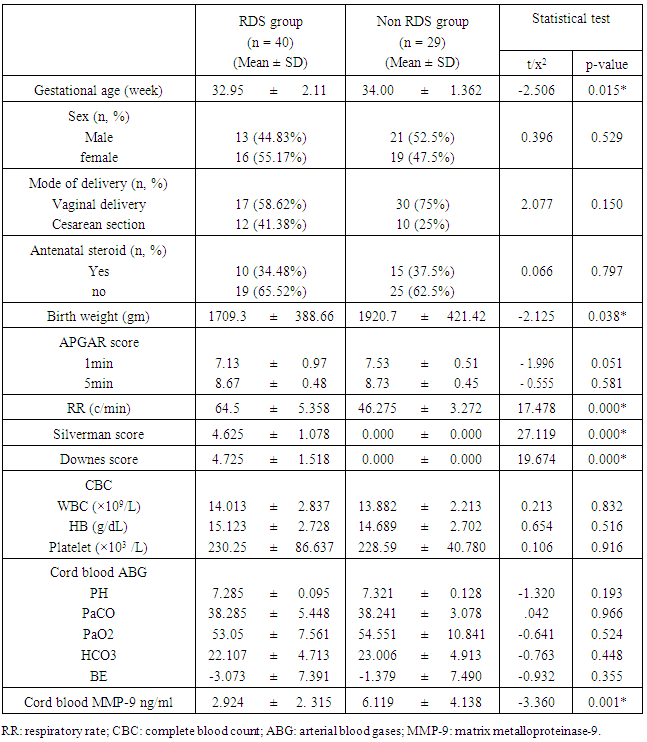
- The current study included 69 preterm neonates (34 males and 35 females). Their gestational age ranged between 29-36 weeks. Forty of them had developed RDS while 29 did not developed RDS. Table 1 shows the general characteristics, laboratory investigations of them. Preterm infants who developed RDS had significantly lower gestational age, birth weight and lower level of cord blood MMP-9 than those without RDS (p < 0.05).
|
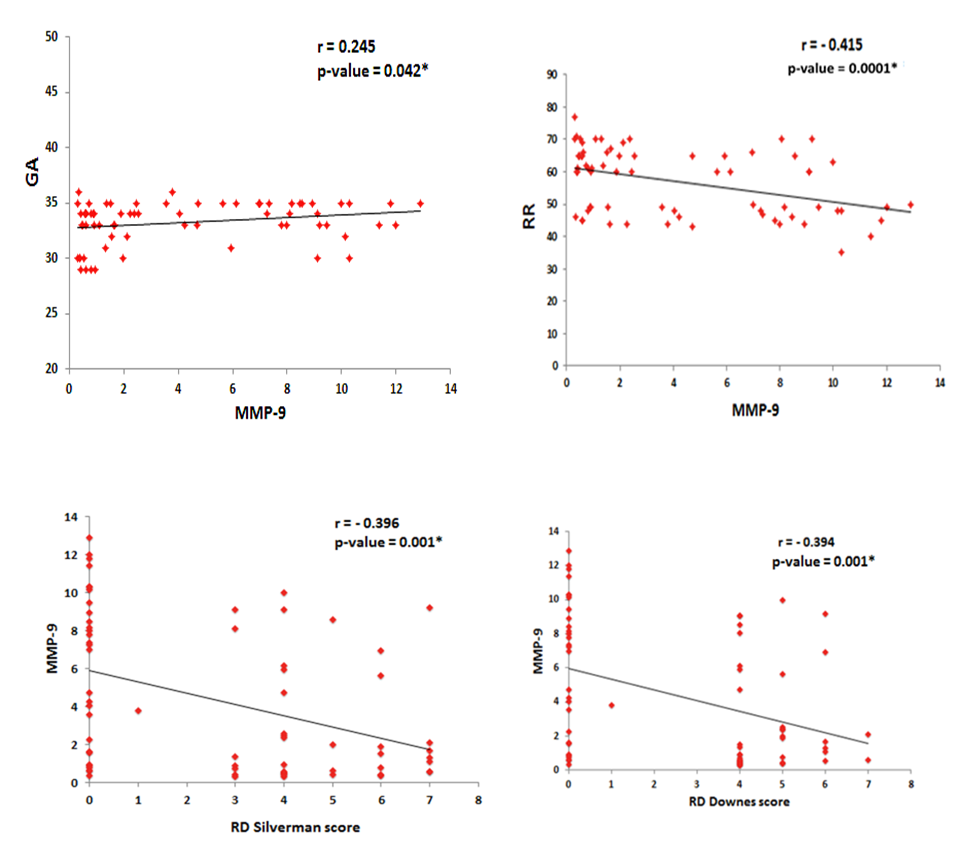 | Figure 1. Correlations between cord blood MMP-9 level, gestational age (GA), RR and RD scores in preterm infants |
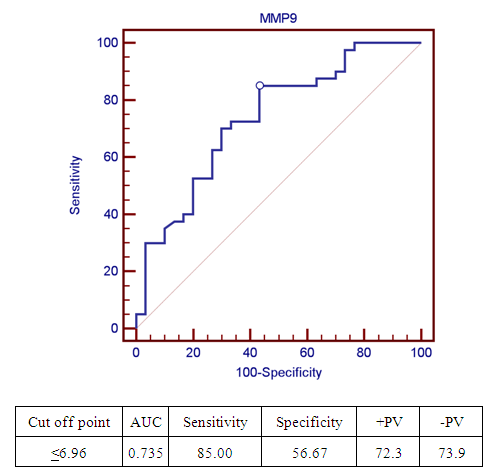 | Figure 2. Receiver operating characteristic curve (ROC) of cord blood MMP-9 level for diagnosis of RDS in preterm infants |
|
4. Discussion
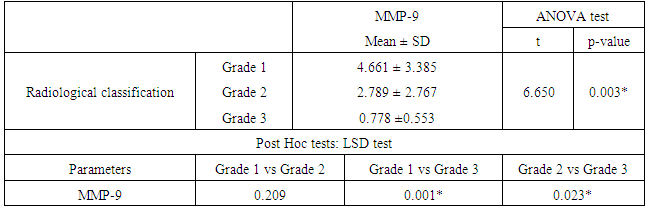
- The matrix metalloproteinase (MMP) family of extracellular proteinases regulates many developmental and physiological events. In vitro studies suggest that MMPs can affect fundamental cellular processes such as proliferation, survival, migration, and morphogenesis. These are the key processes of development. Because MMPs can degrade extracellular matrix (ECM) molecules, their main function has been presumed to be remodeling of the ECM. They are thought to play important roles during embryonic development, as ECM remodeling is a critical component of tissue growth and morphogenesis [22].Development of the human lungs progresses through pseudoglandular, canalicular, saccular and alveolar stages. The distal airspaces are expanding during the late gestation and continue to expand postnatal. Adequate lung development is the major factor that decisively affects the postnatal outcome in the perinatal period [23]. MMP have the ability to degrade most of the stromal components, so they have a key role in degradation of intracellular space, as well as in remodeling of lung interstitium, epithelial basal membrane and blood vessel endothelium [24]. Degradation of the ECM by MMP-9 occurs in both physiological and pathological conditions leading to modulation of growth and development, vascularization and tissue remodeling processes [25]. Fibroblast growth factor, vascular endothelial growth factor, and their receptors are released by MMP cleavage of the extracellular matrix [26]. Mesenchymal MMP-9 expression is important not only for alveolarization but also for angiogenesis [27]. RDS is a squeal of developmental insufficiency of surfactant production and structural immaturity of the lungs. It is well known that RDS is strongly related to the gestational age. The risk for developing RDS is more than 60% at 29 weeks, 20% at 34 weeks and less than 5% at 37 weeks or later [28]. Since MMP-9 is involved in ECM degradation, its role has been studied in several lung conditions. In recent years, MMP-9 has been extensively studied as a key player in airway inflammation and remodeling [29]. On the lights of these data, our study aims to explore the value of cord blood level of MMP-9 as a predictive of RDS development in preterm labors and correlate its level with the radiological finding in the 1st hour of life. Our study demonstrated that cord blood MMP-9 level was significantly lower in patients developed RDS (p<0.001) with significant positive correlation with gestational age (P= 0.045). Cord blood MMP-9 level has a sensitivity rate 85% and specificity rate 56.67% for predicting RDS in preterm infants at a cut-off value 6.96ng/ml using the ROC curve.Our study confirms the results of previous studies by Matoba et al [30] reported that MMP-9 is one of the cord blood biomarkers that decreased in preterm birth. Kaukola et al [31] found that Cord blood MMP-9 was related independently to RDS. Infants without RDS had higher MMP-9 levels in cord blood than did infants with RDS. Kraljevic et al [32] study showed significant mesenchymal MMP-9 expression in all stages, with a peak expression in the saccular stage. MMP-9 play important role in proliferation and apoptosis of the mesenchymal cells of canalicular stage which is an important step for formation of definite structures within the stroma of the lung parenchyma during lung development. Additionally, MMP-9 expression is important for primitive alveoli differentiation. Low cord blood MMP-9 levels in RDS infants may be the expression of a biological immaturity of the fetus, independently of gestational age.Increased MMP‑9 activity is associated with better pulmonary outcomes in infants with RDS, suggesting that MMP‑9 plays a protective role [33]. In our study, there was significant positive correlation between cord blood MMP-9 and degree of RD. Regarding the radiological scoring of RDS, preterm infants with more sever radiological findings (grade III) had significant lower level of cord blood MMP-9. A study of 1059 preterm infants with birth weights <1000 g detected a significant negative correlation between MMP‑9 levels on day one of life and administered FiO2 on day three [34]. MMP-9 is available biomarker and can be measured by different technologies such as zymography, ELISA and luminex [35]. MMP-9 level is extensively studied in many pathological condition but there is limited data about its level in physiological condition for evaluation of lung maturity [36]. Adequate lung development is the major factor that decisively affects the postnatal outcome in the perinatal period. Development of strategies for risk stratification and prediction of morbidity in preterm infants include identification of simple, rapid, and safe markers of lung maturity to allow early interventions and prevent morbidity. There is little information on the clinical usefulness of cord blood MMP-9 in diagnosis and risk stratification of RDS in preterm infants. Most of the studied cord blood biomarkers for prediction of RDS in preterm infants are related to surfactant synthesis. Cord blood MMP-9 may provide additional information about the morphological changes of the lung rather than surfactant production. For the best of our knowledge, this is the first study to explore the correlation between cord blood MMP-9 and radiological findings in preterm infants. Our results provide important information that could be used to guide additional studies aimed at determining risk stratification of RDS in preterm labor.
5. Conclusions
- Assessment of cord blood MMP-9 through cordocentesis in high risk pregnancies susceptible for preterm labor can give a prediction for those who have immature lung and help to predict the severity of RDS and arrange for proper management. Further studies are needed to clarify if cord blood MMP-9 can be used as a prognostic marker in premature infants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML