-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2015; 5(3): 39-44
doi:10.5923/j.cmd.20150503.02
Value of New Modification of Tuberculosis Score in Diagnosis of Childhood Pulmonary Tuberculosis
Hussein Koura1, Abdel-Hamead A. F. Mohammed2
1Pediatric department, Faculty of Medicine-Diametta Al-Azhar University
2Clinical pathology department, Faculty of Medicine- Assiut, Al-Azhar University
Correspondence to: Abdel-Hamead A. F. Mohammed, Clinical pathology department, Faculty of Medicine- Assiut, Al-Azhar University.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
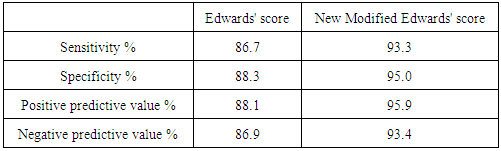
The difficulties in making an exact diagnosis of pulmonary tuberculosis in children have led to the development of different approaches for diagnosis. The aim of this study was to compare the validity of Edwards' score and a new modified Edwards' score in the diagnosis of childhood pulmonary tuberculosis. A cross sectional study was carried out at Al Azher University hospitals. One hundred twenty children were enrolled in the study and were divided to two groups. Tuberculosis group included 60 children with positive pathozyme TB complex test and respiratory symptoms and/orchest-X-ray (CXR) findings that improved using exclusively anti-tuberculosis drugs. Control group included 60 children with significant respiratory symptoms in the form of cough and or difficult breathing or tachypnea of duration not less than 7 days with or without CXR findings and who recovered from their symptoms and/orCXR findings using treatment other than anti-tuberculosis drugs and demonstrated negative pathozyme TB complex test. At enrolment the following investigations were performed: tuberculin skin test (TST), CXR postro-anterior view, complete blood count, ESR and pathozyme TB comp1ex plus test. Edwards' score and a new modified score were applied separately to all enrolled children. Sensitivity and specificity for diagnosis of pulmonary tuberculosis were higher for new modified Edwards' score than Edwards' score (93.3%, 95% versus 86.7%, 88.30% respectively). Also, positive and negative predictive values were higher for new modified Edwards' score compared with Edwards' score (95.9%, 93.4% versus 88.1%, 86.9% respectively). The mean score for diagnosis of pulmonary tuberculosis was higher in new modified Edwards' score than Edwards' score (11.4 ± 0.5 versus 10.3 ± 0.4 respectively). There was agreement between Edward score and new modified Edwards' score in diagnosis of 52 cases as pulmonary tuberculosis. It was concluded that new modified Edwards' score is better than Edwards' score in the diagnosis of childhood pulmonary tuberculosis. It was recommended to conduct a community based study with large sample size to evaluate the validity of new modified score when used on the large scale.
Keywords: Edwards' Score, Childhood Tuberculosis, Pathozyme TB Complex, Tuberculin Skin Test
Cite this paper: Hussein Koura, Abdel-Hamead A. F. Mohammed, Value of New Modification of Tuberculosis Score in Diagnosis of Childhood Pulmonary Tuberculosis, Clinical Medicine and Diagnostics, Vol. 5 No. 3, 2015, pp. 39-44. doi: 10.5923/j.cmd.20150503.02.
1. Introduction
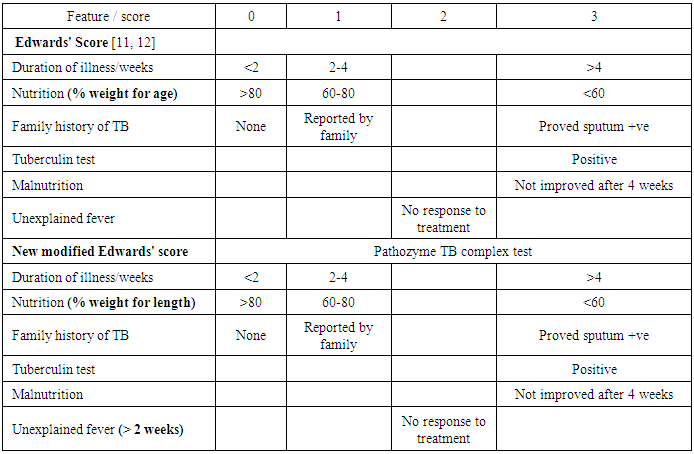
- The diagnosis of childhood pulmonary tuberculosis presents a major challenge, as it is complicated by the absence of a practical "gold standard" [1, 2, 26]. Sputum smear microscopy, often the only diagnostic test available in endemic areas, is positive in less than 10 to 15% of children with probable tuberculosis and culture yields are also low (30% to 40%) [3, 4, 24]. On the other hand serologic tests alone are currently unable to diagnose childhood pulmonary tuberculosis with accuracy [5], sputum-based polymerase chain reaction (PCR) tests have shown variable results and limited utility [6-9] and radiological signs are often difficult to interpret. Owing to the diagnostic limitations discussed above, a variety of clinical scoring systems have been developed to diagnose active tuberculosis in children. A critical review of these clinical scoring systems concluded that they are limited by a lack of standard symptom definitions and adequate validation [1]. Since 1996, WHO has recommended Edwards' score to use in diagnosis of childhood tuberculosis [11, 12]. Many studies were conducted to test the validity of the score in diagnosis of childhood tuberculosis but the results were different [13, 14]. After a long clinical experience in using this score for diagnosis of childhood tuberculosis, we did a modification for two items of the generic score to increase the validity of the score. Weight for length or height (W/L) was used instead of weight for age (W/A) because W/L represents wasting while W/A represents wasting and or shortness. Unexplained fever was determined by duration of more than two weeks to be more accurate. Pathozyme TB complex as "gold standard" test was added to define positive TB and control negative TB groups.This study aimed to compare the validity of Edwards' score and a new modified Edwards' score in the diagnosis of childhood pulmonary tuberculosis.
2. Patients and Methods
- This is a cross-sectional study which was carried out at Al-Azhar University hospitals. One hundred twenty children were enrolled in the study. They were divided into two groups, tuberculosis group and control group. Tuberculosis group included 60 children with positive pathozyme TB complex test [15] and respiratory symptoms and/or chest-X-ray (CXR) findings that improved using exclusively anti-tuberculosis drugs. Control negative non tuberculous group included 60 children demonstrated negative pathozyme TB complex test with significant respiratory symptoms in the form of cough and or difficult breathing or tachypnea of duration not less than 7 days with or without CXR findings and who recovered from their symptoms and/or CXR findings using treatment other than anti-tuberculosis drugs. After enrolment a full history was taken using a predesigned questionnaire and a thorough clinical examination was performed. Nutritional status was assessed according to Waterlow classification [16] by using CDC growth charts [17]. After that the following investigations were performed to all enrolled children: TST by Mantoux technique [18], CXR postro-anterior view, complete blood count by Cell Dyne 1700 and stained blood film, ESR by Westergren method and pathozyme TB complex plus test [15, 25], a commercially available rapid ELISA kit (Omega Diagnostics, Alloa, UK) containing recombinant 38-kDa antigen, and 16-kDa recombinant protein specific for MTB complex. The study was performed according to the manufacturer’s instructions. Diluted (1:50) serum was distributed in micro titer wells and incubated for 60 minutes at 37°C. Unbound serum was removed by washing with a buffer solution. The wells were subsequently incubated with peroxidase labeled anti-human conjugate at 37°C for 30 minutes. After another wash cycle, peroxidase substrate tetra-methyl-benzidine containing hydrogen peroxide was added to the wells and the colorimetric reaction kept in dark at 37°C for 15 minutes thin stop reagent was added. Absorbance values at 450 nm were recorded. Four standards (with 2, 4, 8 and 16 sero unit/ml) were provided to generate a semi-logarithmic reference curve. Because the sera were diluted 1:50, the units extrapolated from the curve were multiplied by 50 to obtain serological units for result interpretation. According to manufacturer’s instructions, a result was considered positive when the level of antibodies in a sample was higher than 200 serological units/ml. The serum samples, positive, negative and cut-off controls included in the kit were tested in duplicate. Results are expressed as the number of serological units of specific IgG per ml and were read from a semi-logarithmic reference curve, which prepared by using the standard solutions included in the kit. After that Edwards' score and a new modified score were applied separately to all enrolled children. For every point a score was given as shown in the table 1.
|
3. Results
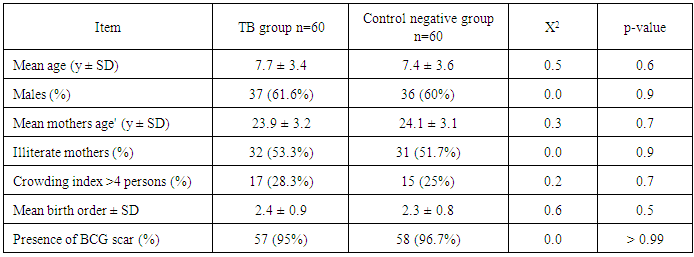
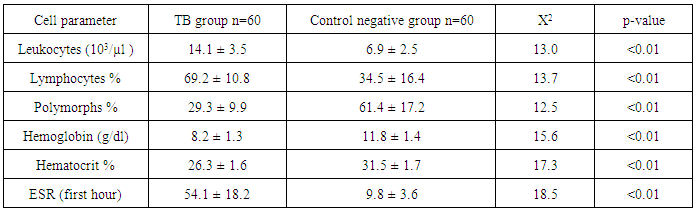
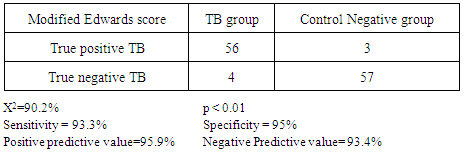
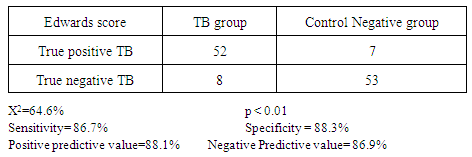
- Tuberculosis group and control negative group were compared regarding demographic characters (table 2). BCG scar was present in 57 (95%) and 59 (96.7%) patients among tuberculosis group and control group respectively (table 2). Total leukocytic count and percentage of lymphocytes were higher among tuberculous group comparing to control negative group and the difference between the two groups were statistically significant (table 3, p<0.01 each of them). Hemoglobin level and hematocrit were lower in the tuberculosis group than the control group (table 3, p<0.01). ESR was higher in the tuberculosis group comparing to the control group and the difference between them was statistically significant (table 3, p<0.01). Sensitivity and specificity for diagnosis of pulmonary tuberculosis were higher for new modified Edwards' score than Edwards' score (tables 4, 5, 6, 93.3%, 95% versus 86.7%, 88.3% respectively). Also, positive and negative predictive values were higher for new modified Edwards' score in comparing to Edwards' score (tables 4, 5, 6, 95.9%, 93.4% versus 88.1%, 86.9% respectively). The mean score for diagnosis of pulmonary tuberculosis was higher in new modified Edwards' score than Edwards' score (figure 1, (11.4 ± 0.5) vs. (10.3 ± 0.4) respectively). There was agreement between Edward score and new modified Edwards' score in diagnosis of 52 cases as pulmonary tuberculosis.
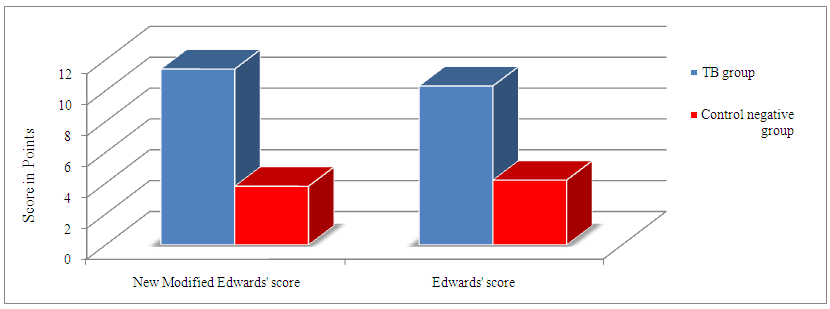 | Figure 1. Comparison between mean TB score in new modified Edwards' score and Edwards' score |
|
|
|
|
|
4. Discussion
- Pulmonary TB can mimic many common childhood diseases. The clinical symptoms in children are cough, fever, wheezing, fatigue, and failure to gain weight. The distinction between recent primary infection and active disease is highly problematic. Childhood PTB are nonspecific and up to 50% of children may be asymptomatic in early stages of the disease. Other aspects like HIV co-infection and malnutrition make difficult the diagnosis of childhood tuberculosis [10]. The diagnosis of tuberculosis in children is complicated with the absence of a practical reference test or gold standard test [26], the inability of pre-adolescent patients to expectorate sputum, the nonspecific clinical presentation, the lower bacillary load often smear negative [27]. Confirmation by culture of Mycobacterium tuberculosis, the gold standard of diagnosis in adult TB, rarely exceeds 30–40% sensitivity [3, 4, 24, 28], even when using gastric lavage (the best specimens for testing for suspected pulmonary TB in children), induced expectoration, use of liquid culture media with indicator of bacterial growth, and polymerase chain reaction (PCR), in addition.Owing to the diagnostic limitations discussed above, a variety of clinical scoring systems have been developed to diagnose active tuberculosis in children. A critical review of these clinical scoring systems concluded that they are limited by a lack of standard symptom definitions and adequate validation [1]. Since 1996, WHO has recommended Edwards' score to use in diagnosis of childhood tuberculosis [11, 12]. Many studies were conducted to test the validity of the score in diagnosis of childhood tuberculosis but the results were different [13, 14]. We introduced a new modification of Edwards' score both in the score features and definition of the cases. Moreover, we followed up the cases of TB groups and control negative groups up to improvement or recovery from symptoms and/or CXR findings. The results of this study showed that new modified Edwards' score had higher sensitivity, specificity, positive and negative predictive values than Edwards' score. Although the differences between the two scores were not as big as expected, these increases cannot be neglected in clinical situations.New modification of Edwards' score was based on two items, assessment of nutritional status and fever (unexplained fever). The use of W/L instead of W/A was based on the fact that stunting is prevalent in developing countries such as Egypt where stunting represents 17.3% among under 5 years [21], so stunted child gets high score by Edwards' score and may be wrongly diagnosed as TB. Specifying of unexplained fever by more than 2 weeks in the new modified score determines a clear cut off for definition of unexplained fever. A comprehensive review of clinical scores for diagnosis of childhood tuberculosis emphasizes on the need to standardize symptom definitions to improve the validity of current scores [10]. Fever is one of essential triad for diagnosis of childhood pulmonary tuberculosis [22] so it is important to get accurate definition when used as item for diagnosis of TB.The impact of these new modifications on the sensitivity and specificity appeared in our results as increase in the sensitivity means improvement in the ability of the score for early diagnosis of suspected TB cases 56 (93.3%) true positive TB cases by modified new score vs 52 (86.7%) by original Keith Edwards scale in TB groups and 3 {5.0%} positive score cases vs 7 {11.6%} in control negative groups respectively. This is of marked benefits in developing countries with limited diagnostic facilities and resources [19]. While impact of these modifications on improvement in the specificity which reflects decrease in the number of false positive cases that diagnosed by score 4 (6.7%) score negative cases vs 8 (13.3%) in TB group in new modified Edwards score and Edwards score respectively). This leads to decrease in the cost and time of treatment when the score applied in large scale. Also modified score can be especially important in patients < 5 years of age, as bacteriological diagnosis is difficult in this group and most cases are treated based on less objective clinical and radiographic evaluation [20]. In our study, Edwards' score was found to have a relatively low specificity (86.7%) and a high sensitivity (88.3%) compared with the 95% and 62% respectively reported from Papua New Guinea [13], mainly because they used all clinical criteria of TB for diagnosis such as extra-pulmonary tuberculosis while in our study, we focused only on criteria for diagnosis of pulmonary tuberculosis. Narayan et al [29] demonstrated sensitivity of 91% and specificity of 88% using Edwards' score. The findings of Edwards' score in a study conducted in Zambia did not agree with results of our study because of the high prevalence of HIV infection there. Several studies evaluated the ability of diagnostic strategies to identify pulmonary TB specifically. For example, the Brazil MOH system, designed specifically for pulmonary tuberculosis [20] has shown a sensitivity of 89% and a specificity of 86%. Marais diagnostic criteria, also focused on pulmonary tuberculosis, demonstrated a sensitivity of 90% and a specificity of 82%, this criteria decreased to 51–56% when children under three years of age and HIV infected children were included [30]. This differences due to variations in the strategies of each study, some are community based study, others are screening studies, and/or retrospective studies. Our study was hospital based diagnostic with follow up.The various definitions and subjectivity of many of the criteria included in the diagnostic approaches make it difficult to compare the diagnostic strategies and the attempts at validation. In addition, clinicians likely vary in how they implement the scoring criteria, thus, making the diagnostic thresholds even less consistent [31]. Follow up the cases with respiratory symptoms and/or chest-X-ray (CXR) findings improved using exclusively anti-tuberculosis drugs for positive groups and non TB medications in control negative group according to diagnosis. This indicate that the use of Pathozyme TB complex test as a gold standard test for new modification is promising and can overcome and correct the bias in the scoring system.In the present work we used more accurate case definition for diagnosis of pulmonary tuberculosis, the pathozyme TB complex test, as gold standard test for definition of TB positive group and negative control group.Pathozyme TB complex test is an immunological rapid ELISA test specific for MTB complex antigens. The test used patient serum with easy blood sample and no need for expectorants or gastric lavage, studies proved high sensitivity for MTB infection with no changes of the results between smear negative and positive TB cases [25].Pathozyme TB complex test appeared to be superior compared with tuberculin skin test (TST) which may give false-positive reactions with non TB mycobacterium infections, previous BCG vaccination, incorrect method of TST administration, incorrect interpretation of reaction and incorrect bottle of antigen used. It may give false-negative reactions in cases of cutaneous anergy, recent TB infection, very old TB infection, very young age (less than 6 months old), recent live-virus vaccination or infection (e.g., measles and chicken pox and HIV), incorrect method of TST administration and incorrect interpretation of reaction [23].
5. Conclusions
- It was concluded that new modified Edwards' score is better than Edwards' score in diagnosis of childhood pulmonary tuberculosis. It was recommended to carry out a community based study with large sample size to evaluate the validity of new modified score when used on large scale and to add other specific techniques for better outcome of the score.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML