-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2015; 5(3): 35-38
doi:10.5923/j.cmd.20150503.01
A Retrospective Study of Pancreatic Lipomatosis and Its Association with Diabetes Mellitus in General Population in North India
Rajesh Sharma1, Manjit Arora1, Puneet Gupta1, Manik Mahajan2, Rattan Paul1, Poonam Sharma3
1Department of Radio-diagnosis and Imaging, ASCOMS Hospital, Jammu University, Jammu, J&K, India
2Department of Radio-diagnosis and Imaging, PGIMS, Pt. B.D. Sharma University of Health Sciences, Rohtak, Haryana, India
3Department of Pathology, GMC Hospital, Jammu University, Jammu, J&K, India
Correspondence to: Puneet Gupta, Department of Radio-diagnosis and Imaging, ASCOMS Hospital, Jammu University, Jammu, J&K, India.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
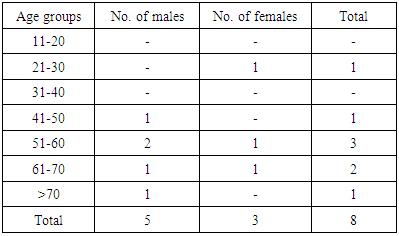
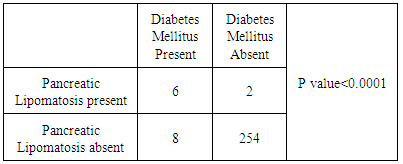
Background/ Introduction: Fatty replacement is a common condition involving the pancreas. Small focal fatty deposits in pancreas are relatively insignificant; however, excessive fat deposition has a pathologic significance and is commonly associated with marked reduction in exocrine function of pancreas. A few studies have investigated the relationship between pancreatic lipomatosis and diabetes mellitus but no such study has been carried out in India according to best of our knowledge. So the objective of this study was to evaluate the incidence of pancreatic lipomatosis in north India and to determine its association with diabetes mellitus. Material and Methods: Multi-Detector Computed Tomography (MDCT) scans of 270 patients who underwent abdominal examination during a period of 2 years at a tertiary care centre in north-west India were retrospectively reviewed. Pancreatic lipomatosis was evaluated in all the patients and their association with diabetes mellitus was analyzed. Results: Among 270 patients, 155 were males & 115 were females. Pancreatic lipomatosis was seen in 8 patients (2.96% cases), even type in 2 and uneven type in 6 cases. In 4 out of 6 (66.7%) patients, the uneven fatty infiltration was of type 1 while in 2 out of 6 (33.3%) patients, type 2 pancreatic lipomatosis was seen. Diabetes mellitus was seen in 14 patients out of 270. Pancreatic lipomatosis was seen in 6 out of 14 diabetic patients (42.8%). Significant correlation was established between pancreatic lipomatosis and diabetes mellitus (p<0.0001). Conclusion: Pancreatic lipomatosis is often an asymptomatic and incidental finding in MDCT seen in 2.96 % cases and significant correlation exists between pancreatic lipomatosis and diabetes mellitus.
Keywords: Computed tomography, Diabetes mellitus, Pancreas, Lipomatosis
Cite this paper: Rajesh Sharma, Manjit Arora, Puneet Gupta, Manik Mahajan, Rattan Paul, Poonam Sharma, A Retrospective Study of Pancreatic Lipomatosis and Its Association with Diabetes Mellitus in General Population in North India, Clinical Medicine and Diagnostics, Vol. 5 No. 3, 2015, pp. 35-38. doi: 10.5923/j.cmd.20150503.01.
1. Introduction
- The pancreas is an exocrine and endocrine organ approximately 15-20 cm long that is related to the stomach, duodenum, colon, and spleen. Fatty degeneration of the pancreas is common with aging; the entire pancreas may be replaced by fat, and the patient may have no clinical symptoms. Fatty replacement of exocrine pancreas, also known as fatty infiltration, lipomatosis, adipose atrophy, or lipomatous pseudohypertrophy is a well-documented benign entity of speculative origin [1]. The exact etiopathogenesis behind fatty replacement is not known; however, several predisposing factors have been suggested. These include obesity, diabetes mellitus, chronic pancreatitis, hereditary pancreatitis, pancreatic duct obstruction by calculus or tumor, and cystic fibrosis [2]. Fatty replacement may be focal or diffuse. Focal fatty replacement is the commonest degenerative lesion of pancreas and has no major clinical significance. Total fat replacement is a rare condition and is associated with pancreatic enzyme deficiency and malabsorption. Following are few subtypes:● Even pancreatic lipomatosis ● Uneven pancreatic lipomatosisThere are four different types of uneven pancreatic lipomatosis. Type 1a (35% of cases) is characterized by replacement of the head with sparing of the uncinate process and peribiliary region; type 1b (36%), by replacement of the head, neck, and body, with sparing of the uncinate process and peribiliary region; type 2a (12%), by replacement of the head, including the uncinate process, and sparing of the peribiliary region; and type 2b (18%), by total replacement of the pancreas with sparing of the peribiliary region [3]. Progressive B-cell dysfunction, in the context of insulin resistance, is a hallmark of type 2 diabetes [4]. Glucose toxicity, ensuing from diabetes related hyperglycemia, has been regarded as a contributor to B-cell damage [5]. In contrast, chronic exposure of the pancreatic islets to non esterified fatty acids (NEFAs) is considered as a potential primary cause of B-cell dysfunction [6]. In obese individuals, increased lipolysis contributes to high levels of circulating NEFAs, whereas liver insulin resistance leads to elevated hepatic output of triglyceride rich particles [7]. When NEFA supply exceeds utilization, non adipose tissues, including the pancreatic islets, start accumulating triglycerides [6], which is aggravated by the simultaneous presence of hyperglycemia [5, 8, 9]. Experimental and autopsy data indicate that fatty infiltration of the pancreas may contribute to a decrease in B-cell mass and function.Few studies have demonstrated the relationship between pancreatic lipomatosis and diabetes mellitus in the literature. However no such study has been carried out from north India according to our knowledge. The aim of this study is to determine the occurrence of pancreatic lipomatosis in patients with and without diabetes mellitus.
2. Material and Methods
- This retrospective study was carried out at a tertiary care centre in north-west India from January 2013 to December 2014. All patients who underwent MDCT for any abdominal pathology during this period were included. Exclusion criteria included excess alcohol intake (>20 units/week), history of hepatitis and/or pancreatitis, recent (>3 months) changes in weight (>5%), and history or current use of glucocorticosteroids and thiazolidinediones. The scans were performed using GE Health Care (Bright Speed Elite 16) computed tomography (CT) system which is modified third generation machine. The CT examinations of patients were performed in a craniocaudal direction. Detector collimation settings of 16 × 0.75 were used with 1.25 mm slice thickness. The data were reconstructed at 0.75-mm slice thickness for multiplanar reformation imaging. A local ethics committee had approved the protocol. Two radiologists independent of each other reviewed retrospectively all the CT scans. All the images were reviewed on a PACS using the continuous axial and coronal reformatted images. The decisions about the CT findings were reached by consensus. Pancreatic lipomatosis was defined as a region of fat attenuation (-50 to 150 HU) on non contrast and post contrast CT. Medical records were reviewed to determine clinical findings, presence of obesity(defined as body mass index of greater than 30), diabetes mellitus, and coexistent diseases and laboratory findings of serum amylase, lipase, and total cholesterol levels.
3. Results
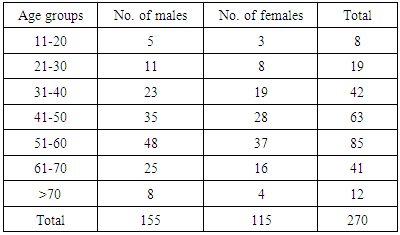
- A total of 270 patients were included in the study. The age of patients varied from 15 years to 75 years. The majority of the patients (31.5 %) belonged to 6th decade (Table 1). Out of 270 patients, 155 (57.4%) were males and 115 (42.6 %) were females with a male to female ratio of 1.35.
|
|
|
|
4. Discussion
- The accumulation of fat in the pancreatic gland has been referred to using various synonyms, such as pancreatic lipomatosis, fatty replacement, fatty infiltration, fatty pancreas, lipomatous pseudohypertrophy, non-alcoholic fatty pancreatic disease and pancreatic steatosis [10]. Pancreatic lipomatosis is becoming an increasing problem worldwide due to the increasing incidence of obesity and diabetes mellitus. Fatty infiltration of the pancreas has been also reported in advanced cases of cystic fibrosis, Shwachman syndrome and Johanson-Blizzard syndrome.Fatty replacement may be focal or diffuse. Focal fatty replacement is the commonest degenerative lesion of pancreas and has no major clinical significance. Total fat replacement is a rare condition and is associated with pancreatic enzyme deficiency and malabsorption. Fatty replacement may be uniform or unevenly distributed in the pancreas.Lee JS et al [11] used ultrasonography to evaluate the fat content of the pancreas. They used the increase echogenicity of the pancreatic body over kidney echogenicity as the index of a fatty pancreas, and they found that a fatty pancreas is related only to the metabolic syndrome. However the role of ultrasound in the diagnosis of pancreatic lipomatosis is very limited because the overlying bowel gas causes obscuration of the pancreas and the fatty infiltration results in increased echogenicity of the pancreatic tissue making its differentiation difficult from normal retroperitoneal fat.Cross-sectional imaging, namely, CT has an important role in the evaluation of pancreatic disease. CT is particularly useful in detecting pancreatic duct obstruction by the calculus or tumor. Fatty infiltration of pancreas is seen on CT evidenced as pancreatic parenchymal soft tissue intermixed with fat. Associated atrophy is also seen in aged individual. Matsumoto S et al [3] reviewed CT scans of 80 cases with uneven fatty replacement. Uneven fatty replacement of the pancreas was classified into two types. In type 1, the posterior aspect of the head of the pancreas was spared from intense fatty replacement. In type 2, the focal area around the common bile duct (CBD) was spared from fatty replacement. Each type was divided into two subgroups on the basis of whether the body and tail of the pancreas showed intense fatty replacement. Twenty-eight patients (35%) had type 1a replacement, 29 (36%) had type 1b replacement, nine (11%) had type 2a replacement, and 14 (18%) had type 2b replacement. In all patients, the anterior aspect of the head of the pancreas showed distinctly lower attenuation at CT. In our study, majority of the patients (75.0%) were type 1 fatty replacement of head, neck and body (Figure 1a, b) and 25.0% were type 2, fatty replacement of head and uncinate process correlating with the study of Matsumoto S et al [3].
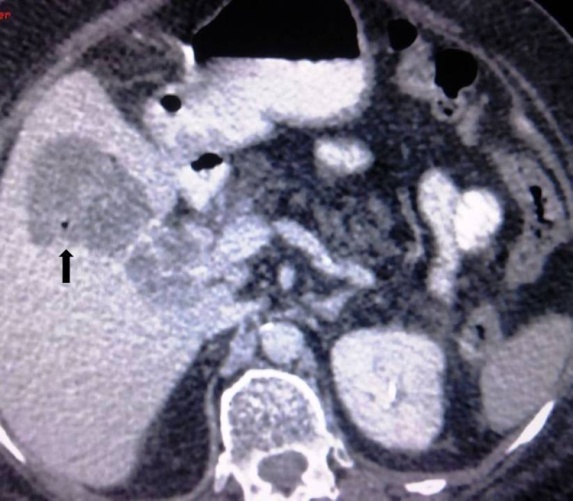
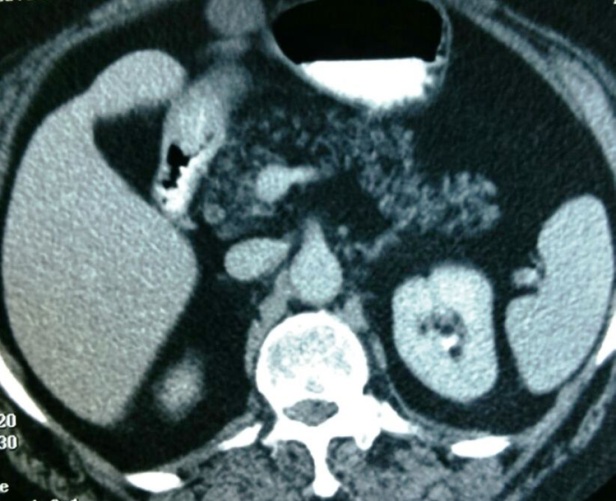 | Figure 1. Axial contrast enhanced CT image showing complete replacement of the pancreatic parenchyma by macroscopic fatty tissue (Even Type of Pancreatic Lipomatosis) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML