-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2015; 5(2): 26-34
doi:10.5923/j.cmd.20150502.03
Role of Paraoxonase Activity and Oxidative Stress in Renal Vascular Damage in Type 2 Diabetes Mellitus
Sabah AM1, Reham S.1, Abdul Baset A. F.2, Eman S.3
1Medical Biochemistry Department, Faculty of Medicine, Alexandria University
2Medical Biochemistry Department, Faculty of Medicine, Tripoli University
3Department of Medicine Alexandria University Hospital
Correspondence to: Sabah AM, Medical Biochemistry Department, Faculty of Medicine, Alexandria University.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The vascular effects of hyperglycemia can be macrovascular complications or microvascular complications in the form of retinopathy, nephropathy and neuropathy. HDL is important in preventing oxidation of LDL; this activity resides mainly in paraoxonase enzyme (PON1) that is closely associated with HDL molecules. The present study aimed at evaluation of the role of PON1 activity and oxidative stress in renal microvascular complications in Type 2 diabetic patients. The study was performed on 75 subjects: Group I: 25 patients of type 2 diabetes mellitus without microvascular complications. Group II: 25 patients of type 2 diabetes mellitus with renal microvascular complications (as evidenced by urinary albumin excretion UAE (mg/24 h) 4.3±1.4). Group III: 25 apparently normal subjects matched in age and gender with group I and II. In all subjects, we examined the activity of PON1, lipid profile and markers of oxidative stress [malondialdehyde (MAD) and Total antioxidant capacity (TAC)]. The results revealed that: triglyceride, total Cholesterol and LDL had statistically high significant levels in group I and II compared to the control group, with higher levels in group II than group I. HDL showed significantly lower levels in group I and II than the control group, with lower levels in group II compared group I. Serum MAD level was significantly elevated in both group I and group II compared to group III and was significantly elevated in group II as compared to group I. There was also a significant lowering of serum total antioxidant capacity in both group I and group II as compared to the control group, and a significant lowering in group II compared group I. Serum basal paraoxonase activity was significantly reduced in group I and group II as compared to the control group, with a significant low activity in group II compared to group I. A significant direct correlation was found between serum paraoxonase activity and serum HDL level with a significant indirect correlation between serum paraoxonase activity and each of serum LDL and total cholesterol levels. Also, a negative correlation was detected between serum paraoxonase activity and TAC. TAC showed a significantly direct correlation with serum paraoxonase activity and HDL, while it showed an indirect correlation with LDL. A significant direct correlation was found between serum MAD level and both serum LDL and total cholesterol levels, meanwhile a significant indirect correlation was found between serum MAD and serum HDL levels. We concluded that oxidative stress together with low PON1activity are predictive risk factors for renal microvascular complications in DM; thereby it is recommended to have therapeutic antioxidant agents that can protect against oxidative stress and support the activity of PON1, this may be of great help in reducing renal vascular damage.
Keywords: Paraoxonase, Diabetes mellitus, Microvascular complications
Cite this paper: Sabah AM, Reham S., Abdul Baset A. F., Eman S., Role of Paraoxonase Activity and Oxidative Stress in Renal Vascular Damage in Type 2 Diabetes Mellitus, Clinical Medicine and Diagnostics, Vol. 5 No. 2, 2015, pp. 26-34. doi: 10.5923/j.cmd.20150502.03.
Article Outline
1. Introduction
- Diabetes is a group of chronic diseases characterized by hyperglycemia. A lot of effort is exerted to overcome the effect of hyperglycemia including lifestyle modification and the use of various therapeutic agents. The diabetic effects on the vascular system are one of the most damaging complications that make this disease serious. These complications can be macrovascular, resulting in ischemic heart disease, stroke and peripheral vascular diseases; or microvascular leading to retinopathy, nephropathy and neuropathy [1].The diabetes duration as well as the severity of hyperglycemia is associated with increased risk of microvascular and neuropathic complications of Type2 diabetes mellitus. Diabetic nephropathy is the main cause of chronic renal failure globally. [2]Many types of renal cells are involved in the injurious effect of hyperglycemia. The pathological sequence of diabetic renal disease involves hyper filtration together with microalbuminuria then ends by deterioration of renal functions. [3]Reactive oxygen species (ROS) are accused of being a common causative and amplifying factor of this cellular damage. [4, 5] ROS are normal cellular products; they are metabolized and degraded by specific mechanisms. However, in some circumstances e.g. hyperglycemia; they accumulate leading to damage in many tissues. [6]From the most important ROS involved in tissue damage are hydroxyl radical, superoxide anion and hydrogen peroxide. [7] The antioxidant balance of these ROS is performed by several enzymes, including superoxide dismutase and heme oxygenase-1 which are induced by hyperglycemia. [8]Paraoxonase (PON) (aryldialkylphosphatase [EC 3.1.8.1]) is synthesized in the liver. It is important in hydrolysis of aromatic acid esters, organophosphorus compounds and nerve gases. [9] There are three members in the paraoxonase enzyme family PON1, PON2 and PON3; their genes are present on chromosome 7q21-22 adjacent to each other’s. [10] PON1 and PON3 are present in blood associated with HDL that is considered as a carrier for them, while PON2 is expressed in tissues and not detected in blood. [11]HDL was proved to prevent the LDL oxidation; an enzymatic mechanism is suggested to be involved in this function. It was found that the separated paraoxonase from human HDL is capable to decrease the liability of LDL lipid peroxidation. This supports the role played by this enzyme in getting rid of lipid peroxides; and adds a probability that people who have low paraoxonase activity could be liable to develop atherosclerosis more than those with high activity. [12]Besides the anti-atherogenic effects, PON1 performs other functions. [13-15] It was found that HDL-PON can hydrolyze the oxidized phospholipids and can reduce peroxides, therefore protects both LDL and HDL together with cell membranes from the effects of lipid oxidation. [16] Moreover, it was proved that the activity of PON1 is reduced during the antioxidant activity of HDL suggesting that it can be used as an indicator of the antioxidant power of HDL. [17]Malondialdehyde (MDA) is a product of oxidative degradation of polyunsaturated lipids by reactive oxygen species. MDA forms covalent protein adducts that have toxic effects on the cells. [16]
2. Aim
- The present study aimed at evaluation of the role of paraoxonase activity and oxidative stress in renal microvasular complications in Type 2 diabetic patients.
3. Subjects
- The study was performed on 75 subjects that were divided as follows:Group I: 25 patients of type 2 diabetes mellitus (T2DM) without microvascular complications. As evidenced by urinary albumin excretion UAE (mg/24 h) 18 ± 2.6Group II: 25 patients of type 2 diabetes mellitus (T2DM) with renal microvascular complications. As evidenced by urinary albumin excretion UAE (mg/24 h) 43±1.4Group III: 25 apparently normal subjects that were age and gender matched with group I and II
4. Methods
- Venous blood samples were drawn in the fasting state (for at least 10 h) and processed immediately into two tubes; one containing fluoride for estimation of fasting blood glucose. The Second tube containing EDTA for separation of plasma, the tubes were centrifuged at 3000 rpm for 15 minutes, the plasma was separated and stored at - 20°C until analysis.The following parameters were estimated:-
4.1. Glycosylated hemoglobin (HbA1c) [18]
- This procedure utilizes a weak binding cationexchange resin for the rapid separation of glycated hemoglobin A1c from all the other hemoglobins.A hemolyzed preparation of the whole blood is mixed continuously for 5 minutes with a weak binding cationexchange resin. During this time, HbA0 binds to the resin. HbA0 consist of all the other hemoglobins except A1c which remains in solution. After the mixing period, a filter is used to separate the supernatant containing the A1c from the resin. The percent glycohemoglobin is determined by measuring the absorbance at 415 nm of the A1c fraction and the total hemoglobin fraction. The ratio of the two absorbances gives the percent of HbA1c.
4.2. Malondialdehyde (MAD) Assays: [19]
- Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) in acidic medium at temperature of 95°C for 30 min to form thiobarbituric acid reactive product which can be detected colorimetrically.
4.3. Total Antioxidant Capacity (TAC) Assays: [20]
- The determination of the antioxidant capacity is performed by the reaction of antioxidants in the sample with a defined amount of exogenously provide hydrogen peroxide (H2O2). The antioxidants in the sample eliminate a certain amount of the provided hydrogen peroxide. The residual H2O2 is determined colorimetrically by an enzymatic reaction which involves the conversion of 3,5,dichloro–2–hydroxyl benzensulphonate to a colored product (Biodiagnostic , Egypt).
4.4. Assay of PON1 Activity: [21]
- This was measured by adding serum to Tris buffer (100 mmol/L, pH 8.0) containing 2 mmol/L CaCl2 and 5.5 mmol/L paraoxon (O,O-diethyl-O-p-nitrophenylphosphate; Sigma Chemical Co). The reaction was monitored for 3 minutes at 405 at 25°C and the change in absorbance was recorded. The non-enzymatic hydrolysis reaction was corrected by taking reagent blank containing water instead of the sample. Enzyme units per ml were calculated as follows:Unit of Enzyme/ml = Change in Absorbance/min / 1.31 (where 1.31 is the molar extinction coefficient of phenol)
4.5. Statistical Analysis
- The data were analyzed using the statistical package SPSS version 15. The descriptive statistics were computed to summarize the mean and standard deviation for quantitative variables and percent for quantitative variables. T test and Anova (analysis of variables) followed by post hoc LSD test (multiple comparison) were used. P value < 0.05 was considered significant. Linear Correlations were done to detect the relation between variances.
5. Results
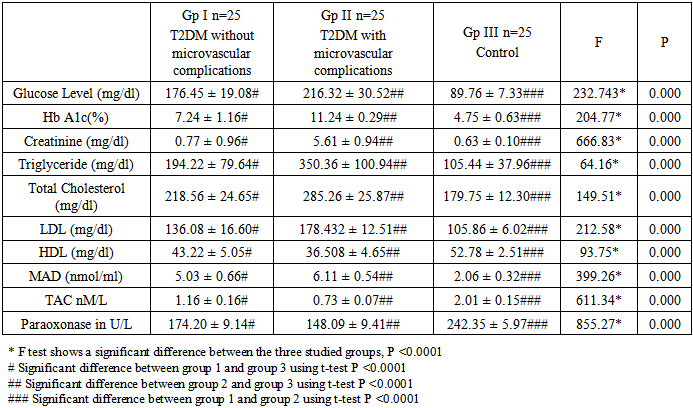
- From table 1 serum glucose level and HbA1c and creatinine showed significant elevation in group I and II compared to the control group (P >0.0001), with significantly higher levels in group II than group I (P >0.0001). Similarly, triglyceride, total Cholesterol and LDL showed statistically high significant levels in group I and II compared to the control group (P >0.0001), with more higher levels in group II than group I (P >0.0001). Regarding HDL it was found to have significantly lower levels in group I and II than the control group (P >0.0001), with more lower levels in group II compared group I (P >0.0001).
|
|
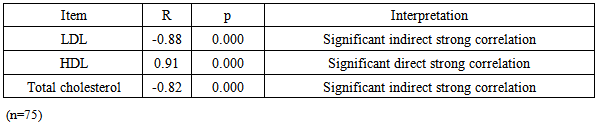
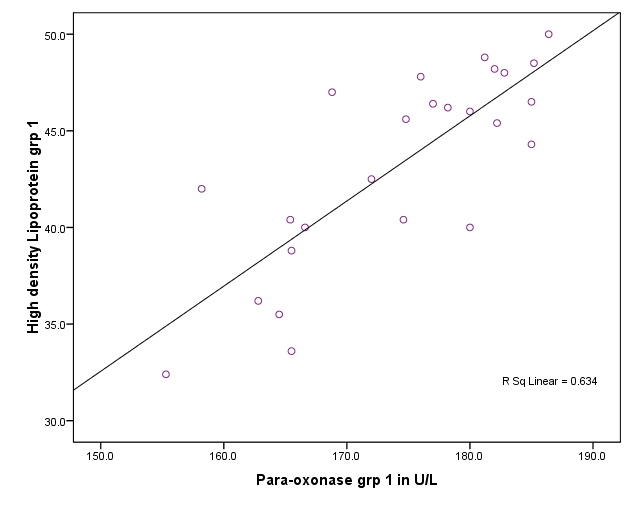 | Figure 1. Correlation between paraoxonase activity and HDL level in group I,II and III |
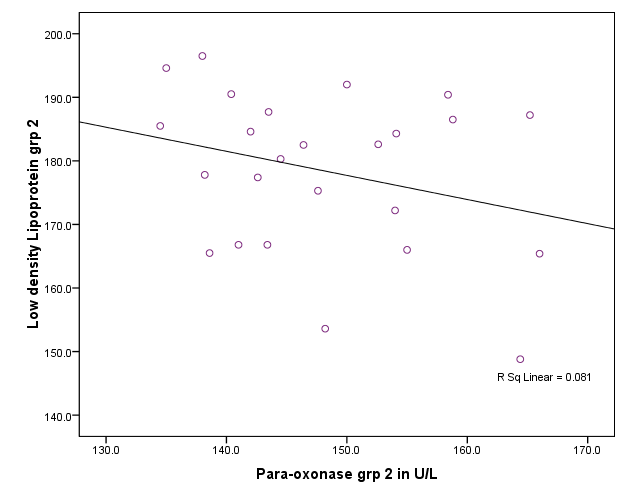 | Figure 2. Correlation between paraoxonase activity and LDL level in group I,II and III |
|
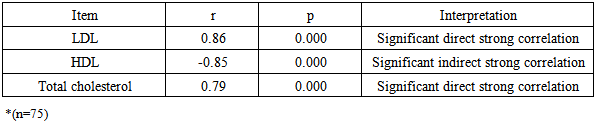
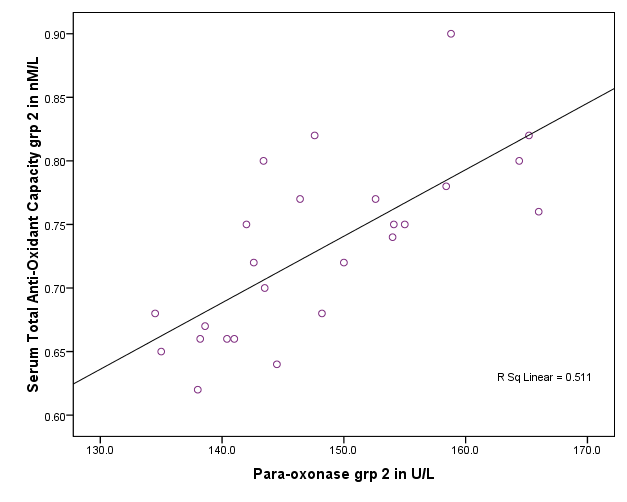 | Figure 3. Correlation between paraoxonase activity and total serum antioxidenet capacity in group II |
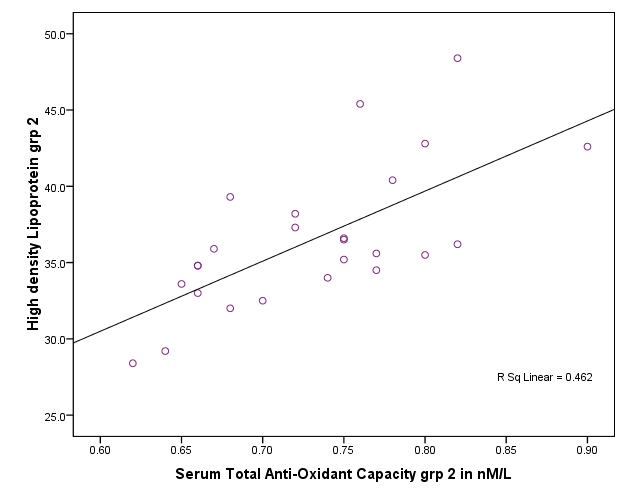 | Figure 4. Correlation between total antioxident capacity and HDL in group II |
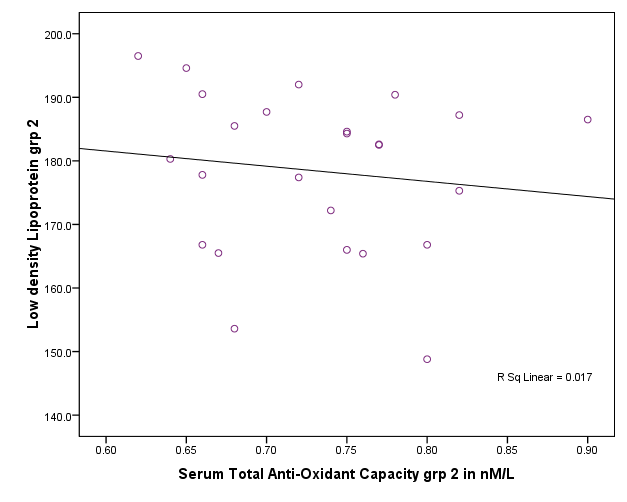 | Figure 5. Correlation between total antioxident capacity and LDL in group II |
6. Discussion
- There is an evident increase in incidence of diabetic chronic complications with high frequency of micro and macrovascular complications in particular that are difficult to be controlled. [22]Chronic renal failure (CRF) is a clinical condition which is accompanied by rapid vascular disease deterioration. [23] Unfortunately, advanced renal disease is commonly seen in diabetic patients because diabetic nephropathy often shows no symptoms except in late stages. Diabetic nephropathy starts by microalbuminuria and ends by macroalbuminuria, which is accompanied by severe and rapid reduction in renal function that may lead finally renal transplantation. [24]Oxidative stress occurs when there is an imbalance between the level of production of reactive oxygen species and the capacity of the antioxidant defense systems. [25] Oxidation of lipids and lipoproteins especially low-density lipoprotein, is considered an important oxidative processes [26], that results in vascular wall damage due to its role in developing atherosclerosis. [27]One of the most important protective factors against arteriosclerosis is HDL. [28] Paraoxonase (PON1) is found exclusively associated with HDL in serum and seems to protect LDL from destruction of its phospholipids. [29] This is why PON1 in particular was investigated in the current study and not the other 2 members of PONs. Low PON1 activities were observed in atherosclerotic and hypercholesterolemic patients [30-33].The present study was designed to evaluate the role of oxidative stress as monitored by the serum total antioxidant capacity and serum MAD levels as well as the role of serum paraoxonase 1 activity in the occurrence of microvascular complications among type 2 diabetic patients.The present study revealed a significant elevation of serum MAD level in patients with type 2 diabetes mellitus as compared to the healthy control subjects. There was also a significant increase in serum MAD level of type2 diabetic patients with microvascular complications in comparison with those without microvascular complications. On the other hand, there was a significant lowering of serum total antioxidant capacity as well as basal serum paraoxonase1 in type2 diabetic patients as compared to the healthy control subjects together with a significant elevation between type2 diabetic patients with microvascular complications in comparison to those without such complications.The above listed results of a condition of oxidative stress are in agreement with those proven by other studies that concluded that oxidative stress is largely involved in the pathogenesis of several diseases, such as DM; and similarly reported a significant reduction of basal serum paraoxonase in diabetic patients as compared to the healthy subjects. [34-36] Moreover, the present study showed that patients with type 2 diabetes mellitus had a significantly high levels of hemoglobin A1C, serum triglyceride, serum cholesterol and serum LDL levels in comparison with their respective healthy control subjects. Such results are coincident with those of Letellier et al [37] who reported the presence of significantly elevated serum triglycerides, total cholesterol and LDL in type2 diabetic patients in comparison to the healthy control subjects.Previous studies reported that PON1 activity may differ among normal individuals, but subjects showing low activity are more liable to develop lipid oxidation and diseases resulting from oxidative stress more than individuals with normal or high activity of PON1. [38, 39] In agreement with our results, Sztanek et al, [40] demonstrated low PON1 activity in hemodialysis patients. Their results confirmed the concept stating that low PNO1activity is associated with reduction in its role as an anti-atherogenic and consequently leads severe vascular damage that may result in end stage renal disease. They have found higher PNO1 activity and higher HDL levels in patients after kidney transplantation than dialyzed patients; then came to the conclusion that kidney transplantation may play a role in reduction of the oxidative stress.Atamer et al in 2013 [41], investigated the effects of rosiglitazone on paraoxonase activity and metabolic parameters in patients with type 2 diabetes mellitus. They concluded that administration of rosiglitazone, which improves oxidant/antioxidant balance and lipid profile, resulted in better effects on anti-atherogenic paraoxonase activity in patients with poorly controlled type 2 DM. They also found that reduction of the levels of MDA and increases PON1, ApoA-1, and HDL in patients with type 2 DM improved their glycemic control. Kumar in 2012 [42], stated that hypertension is also associated with lower serum levels of HDL concentrations hence could explain alterations in PON1 activities; and concluded that antioxidant therapy may support PON1 activity by increasing the total antioxidant capacity. Therefore, the activity of PON1 could be modified by different biologically active molecules, lipoproteins and their metabolites and even by nutrients and changing the lifestyle manner. [43, 44]Low levels of HDL and low activity of PON1 associated with CKD and hemodialysis had attracted the attention of many investigators due to their link to high risk of cardiovascular diseases. Therefore came the suggestion that if the activity of PON1 is supported this risk will be much reduced. [45-50] In agreement with our findings, previous studies proved that reduction in PON 1 activity is correlated with oxidative stress markers. [50-52] Also, many researchers on CKD had found elevations in oxidative stress and reduction in antioxidants, together with reduction in serum PON1 level and hyperlipidemia [49, 53] but these results alone were not enough to explain the occurrence of hyperlipidemia in these patients. [51-54] Disagreeing with our findings, other investigators reported that reduced PON1 activity is not related to the low HDL level or to the high risk of the cardiovascular diseases, and assumed that cardiovascular insult occurs as a result of low HDL while low PON1 activity has no definite role. [49-51]
7. Conclusions
- From the present study we concluded that oxidative stress together with low PON1activity is considered predictive risk factors for renal microvascular complications in DM. This is why hand on hand with the regular therapy of diabetes, further supply of antioxidants can enhance PON1 activity and combat oxidative stress, hence reduce the vascular diabetic complications including reduce renal microvascular disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML