-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2015; 5(1): 8-15
doi:10.5923/j.cmd.20150501.03
FLT3 Gene Mutation and STAT5 Protein Activation in Egyptian Acute Myeloid Leukemia Patients
Nadia Ahmed Fawzi Barghash1, Nahla Abdel Moneim Hamed2, Eman Ahmed Fathi Shaat1, Iman Hassan Mahmoud Diab1, Samer Mahmoud Zaharan3, Yasmine Mahmoud Nabil Mohamed Kamel1
1Medical Biochemistry, Faculty of Medicine, Alexandria University.
2Internal Medicine (Hematology Unit), Faculty of Medicine, Alexandria University
3Faculty of Pharmacy, Pharous University
Correspondence to: Iman Hassan Mahmoud Diab, Medical Biochemistry, Faculty of Medicine, Alexandria University..
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Acute myeloid leukemia (AML) is a clonal hematopoietic disorder that is frequently associated with genetic instability characterized by a diversity of chromosomal and molecular changes. One of the most common mutations in AML is DNA duplications in the FLT3 gene. FLT3 mutations result in downstream activation of a number of signaling pathways, of these, the STAT5 pathway appears to be most differentially regulated in FLT3-(internal tandem duplication) cells compared to FLT3-WT (wild) cells.FLT3-ITD potently activates the STAT5 pathway. STAT5 induces its target genes such as cyclin D1, c-myc and the anti-apoptotic gene p21, which are important for cell growth. These effects may indicate a role of FLT3-ITD in the aberrant cell growth of leukemia cells. The aim of the current study was to determine the frequency of FLT3 mutation and the level of STAT5 phosphorylation among acute myeloid leukemia (AML) Egyptian patients.Also, to evaluate the relation between FLT3 mutation, STAT5 phosphorylation and treatment outcome.The study was conducted on 50 newly diagnosed adult AML patients presenting to the Hematology Unit of Alexandria Main University Hospital (group I)and 30 age and sex matched healthy volunteers (group II). Group I patients were subjected to history taking, clinical examination, routine investigations, complete blood picture, bone marrow aspirate and immunophenotyping study. FLT3/ITD mutation by Polymerase chain reaction (PCR) and activation status of STAT5 by Western blotting were done to all subjects. The activation status of STAT5 was reevaluated one month from the first day of induction chemotherapy using 3 and 7 regimen. Heterozygous mutation of FLT3-ITDs was found in 50% of AML patients and absent in the control group. FLT3-ITDs was present in 60% of t(15;17) subgroup, and 54.5% of the normal cytogenetic subgroup. The highest frequency in FLT3-ITD cases was in M3 (60%). No significant difference was detected between the FLT3-ITD and FLT3-WT groups as regards response to induction chemotherapy, in responsive or non-responsive patients 50 AML cases or in normal cytogenetic cases (n=33). There was a significant difference in P-STAT5 levels between patients and control subjects. There was a significant reduction in post chemotherapyP-STAT5 levels in responders and non-responders. After induction chemotherapy, all FAB classes showed significant reductions in P-STAT5 levels except M6 class. On the other hand, M2 and M4 classes had shown highly significant reductions in P-STAT5 levels after chemotherapy. There was a significant reduction in P-STAT5 levels after chemotherapy in normal cytogenetic and favorable cytogenetic groups. ROC curve analysis showed that the sensitivity of FLT3-ITD post chemotherapy in AML patients with normal cytogenetics (n=33) increased from 68.18% to 81.82% if it is combined with P-STAT5 levels.Conclusions: FLT3-ITD is a frequent mutation in Egyptian AML patients, however it could not predict for bad response to induction chemotherapy in those patients. Its sensitivity to predict bad response to induction chemotherapy in AML patients with normal cytogenetics is increased when combined with P-STAT5.STAT5 is an important signal transducer in AML, where it is highly phosphorylated in response to a variety of mediators and not only the FLT3-ITD.P-STAT5 is decreased significantly after chemotherapy especially in the good prognostic groups and not in the bad prognostic groups, thus it might account partly for the prognosis in AML patients.
Keywords: Acute myeloid leukaemia, Normal cytogenetic, FLT3, P-STAT5
Cite this paper: Nadia Ahmed Fawzi Barghash, Nahla Abdel Moneim Hamed, Eman Ahmed Fathi Shaat, Iman Hassan Mahmoud Diab, Samer Mahmoud Zaharan, Yasmine Mahmoud Nabil Mohamed Kamel, FLT3 Gene Mutation and STAT5 Protein Activation in Egyptian Acute Myeloid Leukemia Patients, Clinical Medicine and Diagnostics, Vol. 5 No. 1, 2015, pp. 8-15. doi: 10.5923/j.cmd.20150501.03.
Article Outline
1. Introduction
- Acute myeloid leukemia (AML) is a cancer wherein dysregulated differentiation, uncontrolled growth and inhibition of apoptosis lead to accumulation of immature myeloid progenitor cells and progression of oncogenic expression [1]. FLT3 is a membrane-bound receptor with an intrinsic tyrosine kinase domain. [2] The human FLT3 gene maps to chromosome 13 (13q12) and is comprised of 24 exons extending over more than 100 kilobase. [3, 4] It is a member of the platelet derived growth factor-receptor (PDGF-R) subfamily (the so called class III) of receptor tyrosine kinases, characterized by an interrupted kinase domain. [5] A major improvement in our knowledge of the molecular biology of AML has been the recognition that about 30% of patients will have mutation of the FLT3 receptor. [7, 8] There is now strong evidence that the presence of FLT3 mutations can be useful for the discrimination of patients with intermediate cytogenetics who are at risk of a worse clinical outcome. [9] In particular, patients showing a mutation of the FLT3 receptor, have a significantly higher risk of relapse. [9] Additionally, AML patients who do not have FLT3 mutations at diagnosis have been found to occasionally acquire them at the time of relapse. [10]STATs are members of the ubiquitously expressed family of transcription factors. Activation of STATs requires phosphorylation of their tyrosine residues by either the receptors that often display an intrinsic tyrosine kinase activity or by receptor associated kinase. [11]The activated STATs form dimers that translocate into the nucleus and initiate transcription of the growth factor/cytokine responsive genes. The STAT family comprises seven structurally and functionally related proteins: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6. [12]STAT5 exists as two homologous proteins referred to as STAT5A and STAT5B, which are encoded by two different genes [13-15]. They are activated by growth hormone, prolactin, interleukins and colony-stimulating factors. Consequently, they are important for stature, mammary gland development and hemopoiesis. STAT5 also has important immunoregulatory functions [16].STAT5 is well recognized as an oncoprotein and its persistent activation has been identified in a large spectrum of lymphoid and non-lymphoid malignancies [17]. It promotes oncogenesis by modulating several key functions of the malignant cells such as survival, proliferation, migration, invasion, induction of angiogenesis, and evasion of the immune response. It was reported that both STAT5A and STAT5B become constitutively phosphorylated in AML. Constitutive activation of STAT5 has been demonstrated in approximately 66% of the AML cases, [18, 19] and may be attributed to activating mutations in upstream kinases, such as FLT3, [20] KIT, [21] or janus kinase 2 [22] or alternatively be due to autocrine growth factor production [21].Furthermore, the activation of STAT5A and STAT5B is required for AML cell growth and survival [23].Some studies showed that cytokine-independent cellular proliferation of FLT3-ITD transduced cells was mediated by RAS and STAT5 pathways Demonstration of STAT5 activation in FLT3-ITD highlighted that some of the effects of FLT3/ITDs are unique to the mutated receptor, in which in contrast to FLT3-ITD, ligand-induced FLT3-WT activation does not lead to STAT5 activation and no STAT5 DNA binding [24].
2. Aim of the Work
- The aim of the current study was to: determine the frequency of FLT3 mutation and the level of STAT5 phosphorylation among Egyptian AML patients. Also, to evaluate the relation between FLT3 mutation, STAT5 phosphorylation and treatment outcome in AML patients.
3. Subjects and Methods
- The study was conducted on two groups of subjects: Group I: Including 50 newly diagnosed adult AML patients presenting to the hematology Unit of Alexandria main university hospital and group II included 30 age and sex matched healthy adult volunteers. Every patient in (group I) was subjected to: Proper history taking and clinical examination, routine investigations, complete blood picture, Bone marrow aspirate, immunophenotyping study. FLT3/ITD mutationby Polymerase chain reaction (PCR) and activation status of STAT5 by Western blotting were done to all subjects. AML cases received induction chemotherapy with 3 and 7 regimen combining daunorubicin 45mg/m2 intravenously day 1-3 and cytosine arabinoside 100 mg/m2 by continuous infusion over 24 hours daily for 7 days. [25] The activation status of STAT5 by western blotting was reevaluated in AML cases one month from the first day of induction chemotherapy.Patients were regarded to have complete remission (CR) if they had <5% bone marrow(BM)blasts in the BM aspirate, and to have partial response (PR)if they had from 5% to 25% blasts in BM aspirate while patients having more than 25% blasts in BM aspirate were considered to be refractory to induction chemotherapy [26]. All patients that failed to achieve complete remission were grouped together in one group.
3.1. Analysis of FLT3 Gene Mutation [27, 19, 25, 28, 29, 30]
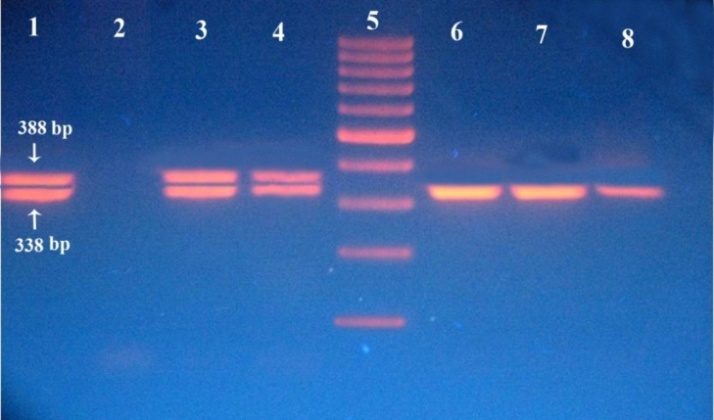
- DNA was purified from the (WBCs in saline) using a spin column protocol using QIAamp DNA blood mini kit. (Qiagen, UK). Extracted DNA was subjected to PCR amplification of exons 14 and 15 of the FLT3 gene. DreamTaq™ Green PCR Master Mix (2x) was used for amplification. (Fermentas, Canada). The master mix contained DreamTaq™ DNA polymerase in 2xDreamTaq™ green buffer, deoxynucleoside triphosphates (dNTPs), and 4 mM magnesium chloride. A pair of primers (forward and reverse) was utilized to amplify exons 14 and 15 of the FLT3 gene. (The midland certified reagent company). Forward primer sequence5´-GCAATTTAGGTATGAAAGCCAGC-3`.´ Reverse primer sequence 5´-CTTTCAGCATTTTGACGGCAACC-3´.PCR was done using the following protocol: Initial denaturation step of 5 min at 95°C, 40 cycles of 30 sec. at 94°C for denaturation, 45 sec. at 50°C for annealing, 1 min at 72°C for extension, and a final extension step of 10 min at 72°C. The products of PCR were electrophoresed on 2% agarose stained with ethedium bromide. A 302 nm ultraviolet transilluminator (Biometra, Germany) was used for visualization of the DNA bands and they were photographed.
3.2. Detection of STAT5 Phosphorylation by Western Blotting [19, 20, 24]
- For western blotting, equivalent concentrations of protein were separated by 10% SDS–PAGE and transferred onto nitrocellulose filter. The filters were then incubated in blocking solution for 2 hrs at room temperature. The filters were reacted with the Affinity-purified rabbit anti-phospho-STAT5 (Y699) antibody (R&D systems, USA) of 1:1000 for 2 hrs. Then the blot was washed three times (5 minutes each) with 1X PBST (0.05% Tween in phosphate buffered saline PBS) and then washed for 10 min with Tris buffered saline (TBS) with shaking. Filters were then incubated with horseradish peroxidase-conjugated secondary antibody (R&D systems) of 1:1000 for 1 h. After the secondary incubation the membrane was washed 3 times (5min each) with TBST (TBS with 0.05%Tween) and then washed again in TBS with shaking. 3,3′-Diaminobenzidine (DAB) substrate solution was prepared, then 30 μl hydrogen peroxide were added. After developing the color of the blot, the reaction was stopped after appearance of the expected bands by pouring out the substrate and rinsing with distilled water repeatedly. As an internal control, Beta Actin antibody (Affinity-purified Sheep Antihuman/ mouse/rat Actin Antibody from R&D systems) was used as control. The antibody detects human STAT5A when phosphorylated at Y694 and STAT5B phosphorylated at Y699. The antibody does not recognize STAT5A/B unphosphorylated at Y694/Y699.
4. Statistical Analysis
- Predictive Analytics Software (PASW Statistics 18) was used. Qualitative data were tested using Chi-square test and Fisher’s Exact test. Quantitative data were described as mean and standard deviation (SD). The distribution of quantitative variables was tested for normality using Kolmogorov-Smirnov test and Shapiro-Wilk test. D'Agstino test was used if there was a conflict between the two previous tests. The data were revealed to be abnormally distributed. Mann-Whitney test was used to analyze two independent populations and Kruskal Wallis test for more than two populations. Wilcoxon signed ranks test was used to compare between two different periods. Correlations between two quantitative variables were assessed using Spearman's correlation. Significance test results were quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level. Receiver operating characteristic (ROC) curve was plotted to analyze a recommended cut off, the area under the ROC curve denotes the diagnostic performance of the test. Area more than 50% gives acceptable performance and area about 100% is the best performance for the test.
5. Results
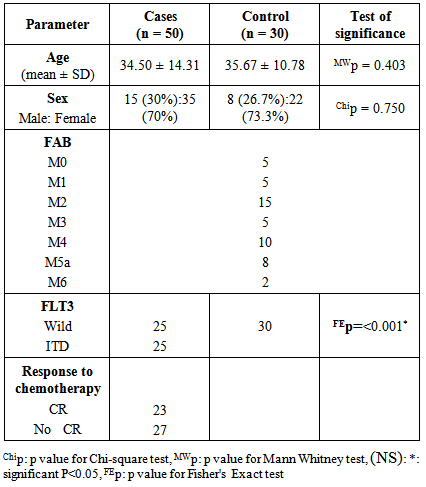
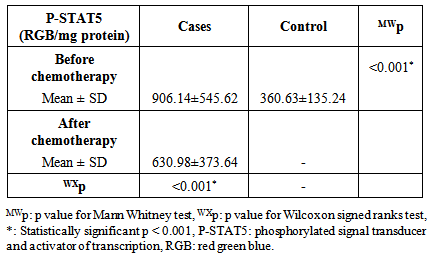
- The diagnostic clinical and laboratory data of the studied patients was presented in table 1. All the control subjects had only the wild band of approximately 338 bp of FLT3 gene while 50% (25/50) of AML cases had a mutant band of approximately 388 bp in addition to the wild band (heterozygous mutation) of FLT3 gene (p< 0.001). (table 1).
|
|
|
|
|
|
6. Discussion
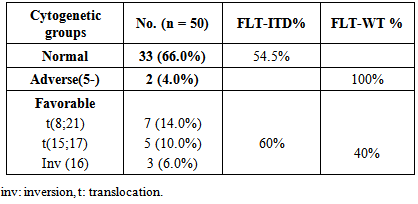
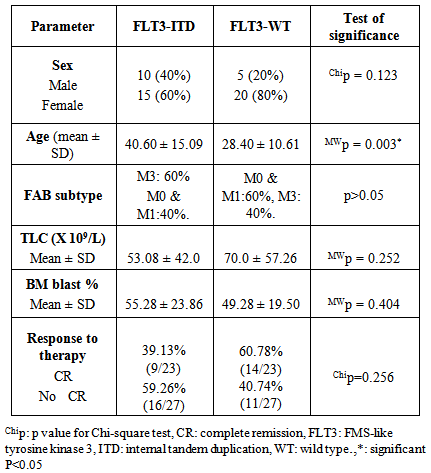
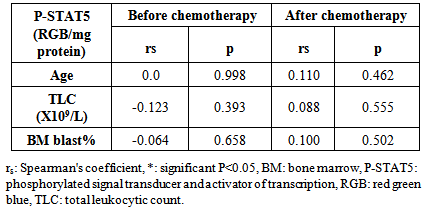
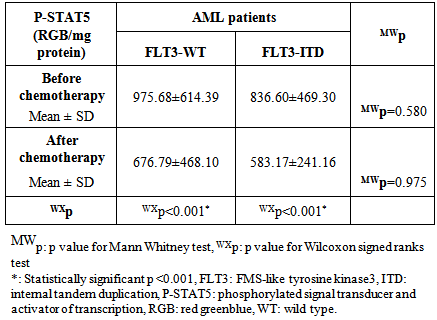
- Acute myeloid leukemia is the most common type of leukemia in adults, yet continues to have the lowest survival rate of all leukemias. [31] AML is often associated with chromosomal translocations and somatic mutations, affecting gene expression in ways that lead to defects in normal programs of cell proliferation, differentiation and survival. [19]Targets of those genetic alterations are most frequently intermediates of intracellular signaling pathways, resulting in aberrant and constitutive signal transduction in AML. [32] Identification of FLT3 mutations in AML has yielded novel approaches to the management of this disease, whether it is through their utility as prognostic factors or their use as a target for directed therapies. [2]In this study, FLT3-ITDs were found in 50% of AML patients. On the other hand, none of the control group showed FLT3-ITD mutation. This finding confirms that FLT3-ITD is an important factor in the molecular pathogenesis of AML, in which it confers a proliferative and survival advantage to hematopoietic progenitors. [33]The high percentage of FLT3-ITD found in our study (50%), could be attributed to the high percentage of AML patients with normal cytogenetics included in the study (66%), low percentage of AML patients with adverse cytogenetics (4%), and the absence of patients with complex karyotype. FLT3-ITDs had the highest frequency among t(15;17) subgroup (60%) followed by the normal cytogenetic subgroup (54.5%), and no ITDs were detected in the adverse cytogenetic group.Most FLT3 studies have demonstrated that the frequency of ITDs is highest in patients with a normal karyotype and in patients carrying a t(15;17), [34] and is lowest in patients with adverse cytogenetics and in those with complex karyotype. [34, 35]In the current study, the age in the ITD group was significantly higher than that in the WT group. This finding is consistent with the concept that the prevalence of FLT3-ITD is highly age dependent. [25] In our work no significant difference was detected between the FLT3-ITD and FLT3-WT groups as regards TLC or BM blast%. According to FAB classification, the highest frequency in FLT3-ITD cases was in M3 (60%). According to the "two hit" hypothesis of leukemogenesis, FLT3 mutations may cooperate with PML/RARA for leukemia development. [36, 37]In this work, no statistically significant difference was observed between FLT3-ITD and WT groups as regards the response to induction chemotherapy, whether patients did or didn't achieve complete remissionin 50 AML cases or in normal cytogenetic cases (n=33).All patients with FLT3-ITD included in the study had both wild and mutant alleles of FLT3 (heterozygous mutation). It has been proven that the presence of the WT FLT3 has the potential to diminish the effect of the FLT3-ITD. [37] Studies have shown that cases that lackedwild-type FLT3 had significantly worse clinical outcome. [38, 39]In addition, and in contrast to wild-type FLT3 signaling, FLT3-ITD potently activates the STAT5 pathway. STAT5 induces its target genes such as cyclin D1, c-myc and the anti-apoptotic gene p21, which are important for cell growth. These effects may indicate a role of FLT3-ITD in the aberrant cell growth of leukemia cells.Our results showed a significant difference in P-STAT5 levels between patients and control subjects. High P-STAT5 levels in AML patients might be due to the constitutive activation of STAT5 in AML, this constitutive activation is essential for cellular transformation and oncogenesis. [24] 72% of AML patients were found to be above the cut-off value for P-STAT5 which was 553 RGB unit/mg protein. There was a significant reduction in P-STAT5 levels in AML patients who achieved or failed to achieve CR post chemotherapy. This reduction may be attributed to the presence of normal WBCs in the peripheral blood cells, after chemotherapy, in which there is no constitutive activation of P-STAT5.The unresponsiveness to induction chemotherapy inspite of the significant reduction in P-STAT5 levels could be due to the involvement of more signaling pathways than just the STAT5 pathway in the expression of the antiapoptotic Bcl-xl protein, eg: nuclear factor kappa beta, it might be that inhibition of only one of these pathways is not enough to reduce the expression of Bcl-xl. [19]P-STAT5 may be a general feature of AML and is not associated with specific parameters since there was no correlation between P-STAT5 levels before or after chemotherapy and age, TLC or BM blast %. No statistically significant difference was observed between the different FAB classes as regards P-STAT5 levels before chemotherapy. After induction chemotherapy, all FAB classes showed significant reductions in P-STAT5 levels except M6 class which is usually associated with bad prognosis. On the other hand, M2 and M4 classes, usually associated with good prognosis, had shown highly significant reductions in P-STAT5 levels after chemotherapy. P-STAT5 might contribute partly to bad prognosis as the alterations in leukemic cell signaling via STAT5 might cause the over expression of the potent oncogene Bcl-xl and allow AML blasts to evade apoptosis. [40]No significant difference was observed inP-STAT5 levels before chemotherapy in the different cytogenetic groups with a significant reduction in P-STAT5 levels after chemotherapy in both the normal cytogenetic subgroup and the favorable cytogenetic group. However the adverse cytogenetic group showed insignificant reduction in P-STAT5 levels post chemotherapy, thus P-STAT5 could be a contributing factor to the bad prognosis usually associated with this group through its antiapoptotic effect.We did not detect significant difference between FLT3-ITD and FLT3-WT cases as regards P-STAT5 levels. The absence of a difference between ITD and WT samples as regards P-STAT5 levels can be explained by several factors: The P-STAT5 seen in WT samples may be due to the large number of other tyrosine kinases and molecules that feed into the STAT5 pathway. [18] A wide variety of hematopoietic growth factors and cytokines, including FLT3 ligand, stem cell factor, granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, Interleukin-3, erythropoietin, and thrombopoietin has been shown to induce STAT5 transactivation. [41]The proportion of FLT3-ITD within a DNA sample is inconsequential unless it is expressed. A study conducted by Seedhouse et al, [42] adjusted the FLT3-ITD levels to take into account FLT3 expression (FLT3-ITD: total FLT3 × total transcript level) which they referred to as ‘adjusted FLT3-ITD level’, these values were significantly associated with P-STAT5 levels.FLT3 mutations are not associated with FLT3 autophosphorylation in all cases, it is likely that the variability in length of the tandem duplications of the different AML cases might be determinative for the dimerization and the subsequent activation of the receptor and the downstream signaling pathways. [19]We measured total P-STAT5 in our study; however there isemerging evidence that the localization of the P-STAT5 may also be important. Bunting et al, [43] demonstrated that P-STAT5 staining by immunohistochemistry in FLT3-ITD cells is predominantly nuclear whereas in FLT3-WT cells it is predominantly cytoplasmic, with only a little nuclear staining. They suggested that biological mechanisms are responsible for the nuclear versus cytoplasmic distribution of P-STAT5 in AML. Cytoplasmic P-STAT5 may not be directed efficiently to the nucleus in the absence of essential interactions provided by the FLT3-ITDscaffold. Alternatively, the hyperphosphorylation of STAT5 mediated by FLT3-ITD may override other mechanisms that may sequester P-STAT5 in the cytoplasm.On the other hand, Harir et al, [44] analyzed the localization of P-STAT5 by immunohistochemistry, but without studying the FLT3-ITD. They suggested that oncogenic activation of STAT5 promotes cytoplasmic localization in myeloid leukemia. Hence both the levels and cellular localization of p-STAT5 may dictate the downstream signaling consequences.To determine if P-STAT5 could serve as valuable prognostic markers in AML patients, ROC curve analysis was performed. For AML patients who achieved CR on induction chemotherapy versus those who didn't. The statistics obtained for P-STAT5 were all below 50%, thus P-STAT5 alone can't predict prognosis in AML patients.Both FLT3-ITD and P-STAT5 were combined and evaluated as prognostic markers in AML patients with normal cytogenetics (n=33) by ROC curve analysis. The sensitivity of FLT3-ITD increased from 68.18% to 81.82% which suggests that the ability of FLT3-ITD to predict bad response to induction chemotherapy in AML patients with normal cytogenetics could be increased if it is combined with P-STAT5 levels.
7. Conclusions
- The sensitivity of FLT3-ITD to predict bad response to induction chemotherapy in AML patients with normal cytogenetics is increased when combined with P-STAT5.P-STAT5 is decreased significantly after chemotherapy especially in the good prognostic groups and not in the bad prognostic groups, thus it might account partly for the prognosis in AML patients. More researches are needed to study the complete pathway for STAT5 phosphorylation and its association with antiapoptotic genes and ROS.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML