Heba Elhakeem1, Reham Sabry1, Lamyaa Ismail Ahmed2, Amany M. Abdallah2, Hayam Hamza Mansour2, Sherif A. Nassib2
1Clinical Pathology Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Internal Medicine Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Heba Elhakeem, Clinical Pathology Department, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Background:Chronic Kidney Disease (CKD) is one of the most important health challenges faced by the world today with new cases being added at an alarming rate. Controlling traditional risk factors has not been effective in bringing down the incidence of the disease. CKD is associated with inflammation, morbidity, poor quality of life, decreased life-expectancy and death. Chronic inflammation is associated with complications and comorbidities frequently seen in CKD. Toll-like receptors (TLRs) are members of innate immune receptors that regulate inflammatory and immune responses and stimulate both immune and nonimmune cells to express inflammatory cytokines and chemokines. Objective:To evaluate the potential role of TLR-2 and proinflammatory cytokines in the pathogenesis of stage 3-4 CKD which could be used to pave the way for prophylactic or therapeutic procedures. Subjects and methods:This study was carried out on 20 patients with stage 3-4 CKD and 10age and sex matched controls. The percentage of CD14+ monocytes expressing TLR-2 and mean florescence intensity (MFI) of TLR-2 on CD14+ monocytes were detected by flowcytometry, while the serum levels of interleukin-6 (IL-6) and IL-18 were measured by enzyme linked immunosorbent assay (ELISA). Results:There was insignificant difference between the percentage of CD14+ monocytes expressing TLR-2 in both patients and control groups(p > 0.05), while there was a highly significant increase in MFI of TLR-2 on CD14+ monocytes, serum IL-6 and serum IL-18 levels in stage 3-4 CKD patients when compared to controls (p ≤ 0.01). There was a significant positive correlation between MFI of TLR-2 on CD14+ monocytes and serum creatinine level (p≤ 0.05, r=0.538) and a highly significant positive correlation between IL-6 serum level and serum creatinine level in patients group (p ≤ 0.01, r= 0.672). Also there was a highly significant negative correlation between MFI of TLR-2 on CD14+ monocyte and estimated glomerular filtration rate (eGFR) (p ≤ 0.01, r=-0.667), and a significant negative correlation between IL-6 and eGFR in patients group (p ≤ 0.05, r=-0.516). Finally, there was a significant positive correlation between MFI of TLR-2 on CD14+ monocytes and serum IL-6 level in patients group (p≤ 0.05 , r = 0.473), while the correlation between serum IL-18 level and serum creatinine level, eGFR, MFI of TLR-2 on CD14+ monocyte and serum IL-6 level in patients group was insignificant (p> 0.05, r =0.168,-0.196,-0.057,-0.010 respectively). Conclusions:This study demonstrated that the expression of TLR-2 on CD14+ monocytes was up regulated in stage 3-4 CKD patients and was associated with inflammatory response as assessed by increased serum levels of IL-6 and IL-18. These findings indicated that TLR-2, IL-6 and IL-18 played a significant role in pathogenesis of CKD.
Keywords:
Chronic kidney disease, Inflammation, Toll like receptor-2, IL-6 and IL-18
Cite this paper: Heba Elhakeem, Reham Sabry, Lamyaa Ismail Ahmed, Amany M. Abdallah, Hayam Hamza Mansour, Sherif A. Nassib, Role of TLR-2, IL-6 and IL-18 in the Pathogenesis of Egyptian Predialysis CKD Patients, Clinical Medicine and Diagnostics, Vol. 4 No. 6, 2014, pp. 118-127. doi: 10.5923/j.cmd.20140406.03.
1. Introduction
Chronic kidney disease (CKD) refers to the progressive and irreversible decline in renal function and is defined as kidney damage for > 3 months based on findings of abnormal structure or function or glomerular filtration rate (GFR) < 60 ml/min/1.73 m2 for > 3 months with or without evidence of kidney damage [1]. Once the diagnosis of CKD is made, staging is based on the estimated glomerular filtration rate (eGFR). Stage 1 being the mildest and usually causing few symptoms, most CKD patients will either be predialysis (stages 3-4) and stage 5 being a severe illness with poor life expectancy if untreated [2]. In CKD, chronic inflammation plays an important role in the disease process. The prevalence of inflammation within the predialysis CKD population is great and is an important indicator of patient health and outcome. Chronic inflammation in the body is measured by the elevated levels of inflammatory markers. There is still confusion about how the levels of some of these markers vary with increasing severity of the disease. Moreover, many of the studies in this regard are based on patients on hemodialysis (HD) [3].Toll-like receptors (TLRs) are a family of transmembrane proteins. They are the major pattern recognition receptors (PRRs) binding to a range of microbial products, often termed pathogen associated molecular patterns (PAMPs). TLRs are members of innate immune receptors which detect infection and regulate inflammatory and immune responses [4].TLR-2 is expressed in both proximal and distal tubules, the thin limb of the loop of Henle and the collecting ducts. Peptidoglycans from gram positive bacteria activate TLR-2. It has a wide range of putative endogenous ligands which include heat shock proteins, high mobility group box1 (HMGB1) and breakdown products of fibronectin, heparan sulfate and hyaluronic acid [4]. A ligand binding to TLR-2 activates the intracellular nuclear factor-κβ (NF-κβ) pathway and enhances the expression of NF-κβ controlled genes such as for inflammatory cytokines and adhesion molecules in monocytes, macrophages, dendritic cells and endothelial cells. Activation of this receptor leads to systemic inflammation in the host [5]. Proinflammatory cytokines are pleiotropic in their nature and impact upon numerous conditions that frequently accompany CKD. While the source (s) of chronic inflammation in CKD can vary, the negative implications of elevated inflammatory markers are clear. CRP frequently used as a clinical marker of inflammation. Interlukin-6 (IL-6) stimulates synthesis of CRP and has been reported as a better prognostic marker than CRP in predialysis CKD populations. Moreover IL-6 is a particularly interesting molecule due to its divergent roles as both a pro-and anti-inflammatory factor [6].IL-18 is a proinflammatory cytokine that is structurally and functionally related to the IL-1 family. It is expressed primarily by macrophages but also by monocytes, dendritic cells and kidney epithelial cells. It plays a role in the innate and adaptive immune response [7]. IL-18 is up regulated in inflammatory states and in addition to its involvement in the immune response, this cytokine has been identified as a mediator of ischemic injury in the heart, brain and kidney. IL-18 has recently been implicated in the pathogenesis of chronic kidney disease. [8]
2. Subjects & Methods
Subjects:The present study was carried out on 20 patients with stage 3-4 CKD and 10 age and sex matched apparently healthy persons as a control group. Patients were obtained from the inpatient of internal medicine department in Alzahraa University hospital during the period from (May 2014 to October 2014). They were 9 females and 11 males with an age ranging from 37 to 61 years old (mean ± SD=49.40 ± 7.90). Written informed consent was obtained from all patients.Exclusion criteria were: (i) patients with signs or symptoms of clinical infection in the previous 3 months; (ii) patients used glucocorticoid or non steroidal anti-inflammatory medication use; (iii) patients with central line insertion or any other invasive procedure within the previous month; (iv) patients with HIV infection; (v) patients with chronic hepatitis B or C infections; and (vi) patients with history of neoplastic, inflammatory or immunological disease. All patients and control groups were subjected to:− Complete history taking.− Thorough clinical examination.− Laboratory investigations as CBC, blood urea, serum creatinine, Na, K, uric acid, eGFR, HCVAb, HBsAg and HIVAb.− Assessment of TLR-2 expression on peripheral blood CD14+ monocytes using flowcytometry.− Determination of serum levels of IL-6 and IL-18 by enzyme linked immunosorbent assay (ELISA).Methods:Venous blood samples were taken from anticubital vein using routine precautions observed in venipunctures and divided into two parts.- Two ml were added to sterile EDTA vacutainers for flowcytometry.- Five ml were taken in plain vacutainers and left to clot for 30 minutes before centrifugation for 10 minutes at 2000 xg. Serum was separated, aliquot and stored at -20℃ for analysis of IL-6 and IL-18. All samples were measured in a single assay to avoid repeated freeze-thaw cycles.Flow cytometric analysis of expression of TLR-2 on CD14+ monocytes: Flow cytometric analysis was performed in fresh samples within 1 hour of blood collection, 100 microliteres of EDTA anti coagulated blood was stained with combination of 10 microliteres conjugated phycoerythrin (PE) labeled monoclonal antibodies for human TLR-2 and 10 microliteres conjugated fluorescene isothiocynate (FITC) labeled monoclonal antibodies for CD14. The mixture was incubated in dark room for 30 minutes then add 1 ml of fix and lyses mixture, vortex the tubes immediately for one second and incubation of the tube was done again for 10 minutes in dark at room temperature. Centrifugation of the tubes at low speed was done for 5 minutes followed by aspiration of supernatant and resuspension of pellet in residual fluid. Two ml of phosphate buffer saline (PBS) were added to each tube, the suspension was centrifuged at low speed. The supernatant was discarded, and then the residual suspension was passed through the flow cytometer. Monoclonal antibodies for TLR-2 and CD14 were supplied by R & D systems Inc (Minneopolis, Minnesota, USA).Data acquisition and analysis were performed on cell quest program of the coulter EPICS XL flow cytometry. Gating on monocytes, 10000 events were acquired and statistical analysis was done by cell quest software. Results were expressed as percentage (%) and mean florescence intensity (MFI) (Fig. 1, 2). 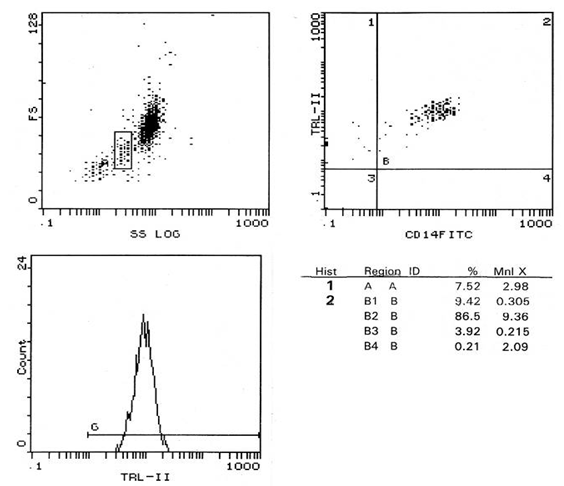 | Figure (1). Percentage of CD 14+ monocytes expressing TLR-2 and its MFI in patients |
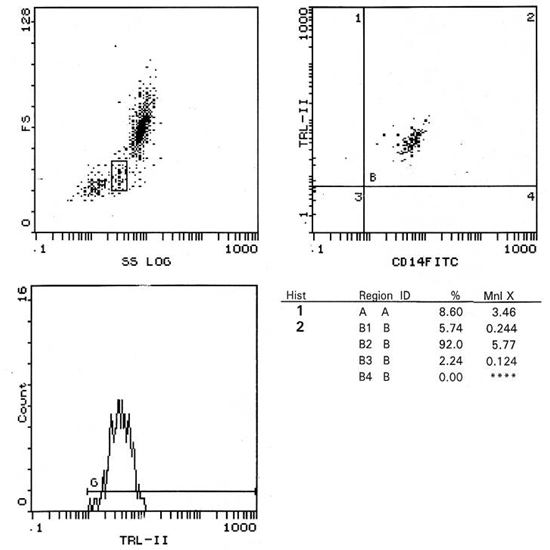 | Figure (2). Percentage of CD 14+ monocytes expressing TLR-2 and its MFI in control |
Analysis of IL-6 concentration in serum by ELISA:Serum IL-6 concentration in serum was analyzed according to the manufacturer’s instructions using ELISA technique, using a complete set of ELISA reader model SLT spectra 216687, with AViBion Human IL-6 ELISA kit, supplied by Ani Bio tech oy. Vanta, Finland. (Catalog number IL06001).Determination of IL-18 concentration in serum by ELISA:Serum IL-18 concentration was analyzed according to manufacturer’s instructions using ELISA technique, using a complete set of ELISA reader model SL spectra 216687, with Human interleukin 18 (IL18) ELISA kit, supplied by glory science. TX, USA (Catalog number 10092).Statistical AnalysisData was analyzed by Microsoft Office 2003 (excel) and Statistical Package for Social Science (SPSS) version 16. Parametric data was expressed as mean ± SD, and non parametric data was expressed as number and percentage of the total.Comparing the mean ± SD of 2 groups was done using paired and unpaired student’s t testMeasuring the mutual correspondence between two values was done using the Spearman correlation coefficient.P value > 0.05 is considered insignificantP value ≤ 0.05 is considered significantP value ≤ 0.01 is considered highly significant
3. Results
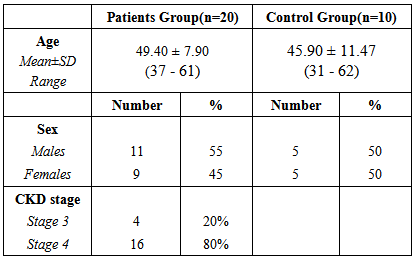
Twenty patients with stage 3-4 CKD and ten apparently healthy individuals were included in our study. The demographic characteristics of the study populations are presented in table 1.Table (1). Demographic data in patients and control groups
 |
| |
|
These data revealed that the percentage of CD 14+ monocytes expressing TLR-2 were not statistically different between patients and control groups (p>0.05), while the MFI of TLR-2 on CD 14+ monocytes showed a highly significant increase in stage 3-4 CKD patients than in control group (p≤ 0.01) (Fig. 3).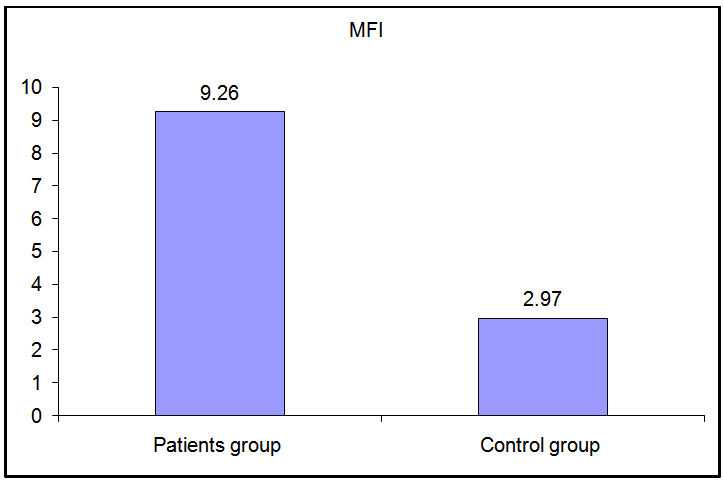 | Figure (3). Comparison of MFI of TLR-2 in patients and controls |
As regards IL-6 and IL-18, there was a highly significant increase in their serum levels in patients group compared to control group (p≤ 0.01). (Fig 4, 5).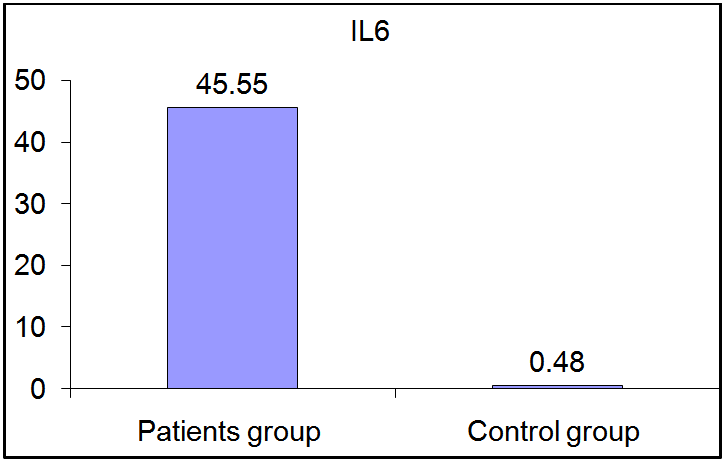 | Figure (4). Comparison of serum IL-6 levels in patients and controls |
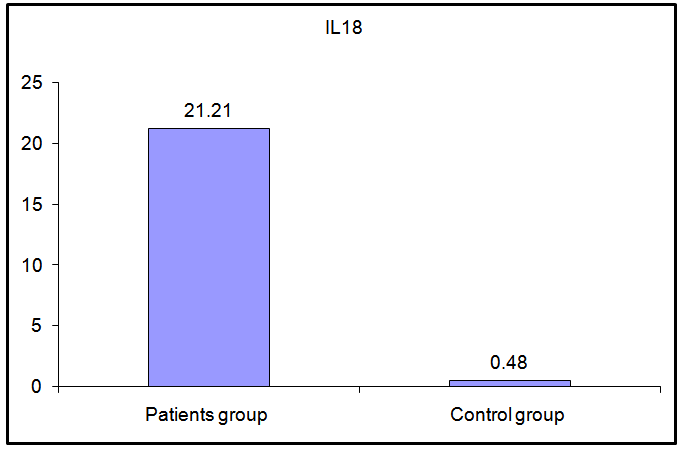 | Figure (5). Comparison of serum IL-18 levels in patients and controls |
Table (2). Comparison between patients and control groups regarding the studied parameters
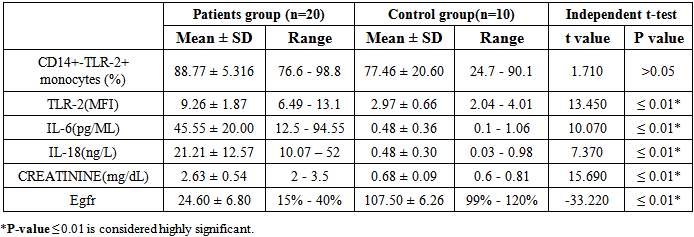 |
| |
|
Table (3). Correlation between the studied parameters in stage 3-4 CKD patients
 |
| |
|
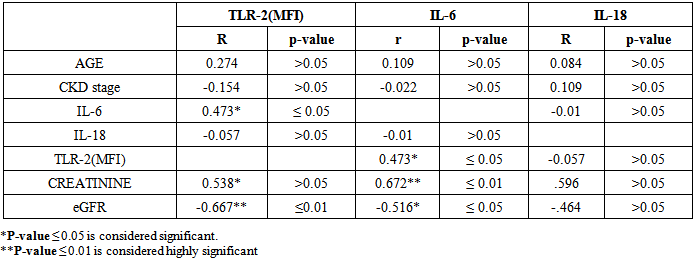
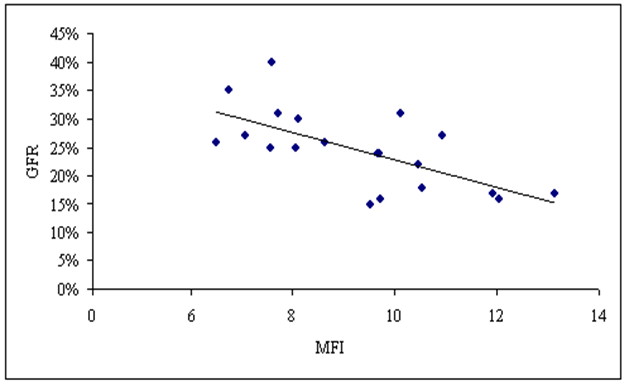
Concerning the correlation between our studied parameters in stage 3-4 CKD patients, there were a significant positive correlation between MFI of TLR-2 and serum creatinine level (P≤ 0.05, r= 0.538), and a highly significant negative correlation between MFI of TLR-2 and eGFR (p ≤ 0.01, r = -0.667) (Fig. 6,7).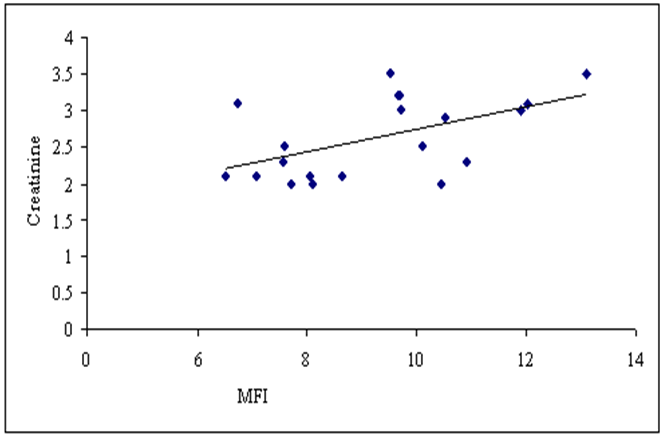 | Figure (6). Correlation between MFI of TLR-2 on CD 14+ monocytes and serum creatinine level in patients group |
 | Figure (7). Correlation between MFI of TLR-2 on CD 14+ monocytes and eGFR in patients group |
As regards correlation between MFI of TLR-2 and proinflammatory cytokine levels, there was a significant positive correlation between MFI of TLR-2 and serum IL-6 level (P≤ 0.05, r= 0.473) (fig. 8), while there was insignificant correlation between MFI of TLR-2 and serum IL-18 level in stage 3-4 CKD patients (p>0.05, r= -0.057).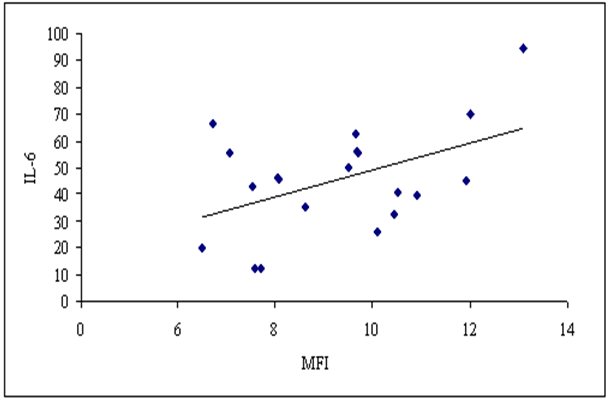 | Figure (8). Correlation between MFI of TLR-2 on CD 14+ monocytes and serum IL-6 in patients group |
As regards IL-6 we detected a highly significant positive correlation between its serum level and serum creatinine level (p≤ 0.01, r= 0.672), and a significant negative correlation between its serum level and eGFR (p ≤ 0.05, r= -0.516) (Fig. 9, 10).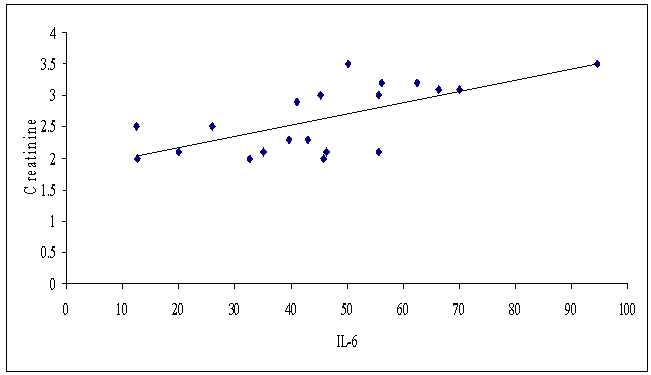 | Figure (9). Correlation between serum IL-6 and serum creatinine level in patients group |
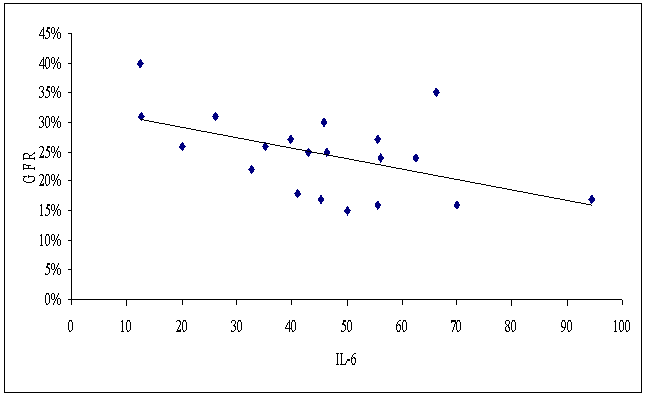 | Figure (10). Correlation between serum IL-6 and eGFR in patients group |
Finally, the correlation between serum IL-18 level and serum creatinine level, eGFR, and serum IL-6 level in patients group was insignificant (p >0.05, r = 0,168,-0.196, -0.010 respectively).
4. Discussion
CKD is a major contributor to morbidity and mortality. Early identification of those at risk of CKD is important and has been facilitated by the routine use of estimating equations for GFR. [7]Chronic inflammation plays an important role in the disease process and high levels of inflammatory markers appear to accompany reduced renal function. Within the predialysis CKD population the prevalence of inflammation is great and is an important indicator of patient health and outcome; high levels of inflammatory markers reflect a chronic inflammatory state associated with reduced serum albumin levels, inadequate response to erythropoietin replacement and greater hospitalization. In addition, in many of the etiologies of CKD, inflammation has a role in their pathogenesis [9].Therefore, the aim of our study is to evaluate the potential role of TLR-2 and proinflammatory cytokines in the pathogenesis of stage 3-4 CKD which could be used to pave the way for prophylactic or therapeutic procedures. Our results showed insignificant difference between the percentage of CD14+ monocytes expressing TLR-2 in both patients and control groups which demonstrated that TLR-2 is normally expressed on CD14+ monocytes. While MFI of TLR-2 on CD14+ monocytes was significantly higher in patients group than in control group which could be explained by the effect of uremia on TLR-2 expression on CD14+ monocytes leading to systemic inflammation in stage 3-4 CKD patients.Tonic interactions between TLR-2 and environmental agonists derived from commensal microbes and endogenous molecules released during cellular necrosis are critical regulators of sterile inflammation affecting the kidney. The resulting inflammation may also lead to excessive tissue destruction and impaired organ function which contributes to the development of chronic kidney disease [10]. Our findings were in agreement with Koc et al., (2011) who found that the percentage of CD14+ monocytes expressing TLR-2 were equal in stage 3-4 CKD patients and controls while MFI of TLR-2 on CD14+ monocytes were significantly increased in patients compared with controls. They speculated that higher MFI values of TLR-2 may be related to chronic low grade monocyte activation in these patients [5].We studied IL-6 as a marker of inflammation in our work. Our data detected a highly significant increase in serum levels of IL-6 in stage 3-4 CKD patients than in controls.Transient increases in IL-6 which are well regulated by an effective anti-inflammatory response are not detrimental, but when IL-6 is chronically elevated and represents an inflammatory milieu its concentrations are associated with poor outcome and are highly predictive of mortality in CKD patients [11]. IL-6 has been implicated in the breakdown of muscle protein in other cachectic populations and related to markers of wasting in CKD patients [12]. Our results were supported by Stenvinkel et al., (2005) who reported that basal levels of IL-6, tumour necrosis factor- alpha (TNF-α) and IL-10 were frequently elevated in CKD, especially in end stage renal disease (ESRD) [13]. Also Koc et al., (2011) detected a significantly higher levels of serum IL-6 in stage 3-4 CKD patients when compared to control group [5].As regards IL-18, it is thought to be one of the mediators of injury in ischemic acute kidney injury (AKI) [14, 15]. AKI is itself a risk factor for future CKD, but there is no reliable means of determining who will recover entirely and who will be left with some kidney impairment following an episode of AKI [16].Our study tried to assess the role of IL-18 in the pathogenesis of stage 3-4 CKD. The results showed a highly significant increase in IL-18 levels in the serum of patients with stage 3-4 CKD in comparison with controls. This might be explained by the proinflammatory characteristics of IL-18, such as increases in cell adhesion molecules, nitric oxide synthesis and chemokine production. It participates in the T-helper-1 (Th-1) paradigm. This property of IL-18 is due to its ability to induce interferon-gamma (IFN-γ) either with IL-12 or IL-15. Without IL-12 or IL-15, IL-18 does not induce IFN-γ but it playas role in Th2 diseases. Similar to several cytokines, the therapeutic focus on IL-18 has shifted from its use as an immune stimulant to inhibition of its activity [17].Our study was supported by Rhee et al., (2013) who have shown that circulating IL-18 levels and renal tubular cell IL-18 receptor expression are significantly increased in patients with CKD [18].Another study was done by Zubowska et al., (2013) to assess the usefulness of IL-18 in the detection of chronic nephropathy in asymptomatic phase of complications in children after anticancer treatment. They concluded that IL-18 seems to be a reliable and accurate marker of chronic nephropathy, especially tubulopathy in those children [19].Meldrum et al., (2012) pointed out that IL-18 has recently been implicated in the pathophysiology of obstructive renal injury which cause renal dysfunction in both adults and children, leading to progressive tubulointersitial fibrosis and apoptotic cell death [8].In the other hand, we studied the correlation between uremia (creatinine, eGFR) and the studied markers (TLR-2, IL-6, IL-18) in stage 3-4 CKD patients. Our results detected a significant positive correlation between MFI of TLR-2 and serum creatinine levels and a highly significant negative correlation between MFI of TLR-2 and eGFR. Our findings provide evidence that systemic activation of the innate immune system can increase inflammation and injury within the kidney or aggravate preexisting kidney disease. [20] As regards the correlation between IL-6 and uremia, we found a highly significant positive correlation between serum IL-6 level and serum creatinine level and a significant negative correlation between it and eGFR. We explained these results by the prevalence of inflammation within the predialysis CKD population. However, in another studies the relationship between the level of inflammation (CRP or inflammatory cytokines such as IL-6 as surrogate markers), and eGFR has not been found to correlate [21, 22]. We also investigated the correlation between TLR-2 expression on CD14+ monocytes and IL-6 in CKD patients. We found a significant positive correlation between MFI of TLR-2 and serum level of IL-6. This might be explained by that TLR-2 is characterized by an extracellular ligand binding domain, single transmembrane domain and intracellular domain. Upon ligand binding, subsequent down stream signal transduction events lead to transcription of pro-inflammatory chemokines and cytokines such as IL-6 [23].Our findings were in agreement with Koc et al., (2011) who found that serum Il-6 level correlated with MFI of TLR-2 in stage 3-4 CKD patients. They concluded that the correlation between inflammatory parameters and TLR-2 expression in CKD might be related to several factors, such as activation of monocyes through uremic toxin retention, chronic stimulation by angiotensin II or increased intestinal permeability to lipopolysaccharide (LPS) as a result of fluid overload [5].Finally, we also made a correlation between serum IL-18 level and serum creatinine level, eGFR, MFI of TLR-2 on CD14+ monocyte and serum IL-6 level in patients group which showed no statistical significance.To the best of our knowledge, no studies have been published concerning the correlation between serum IL-18 level and serum creatinine level, eGFR, MFI of TLR-2 on CD14+ monocyte and serum IL-6 level in stage 3-4 CKD patients. In conclusion: our study showed that the expression of TLR-2 on CD14+ monocytes was up regulated in stage 3-4 CKD patients and the expression pattern of TLR-2 was associated with inflammation as assessed by increased serum levels of IL-6 and IL-18. These findings indicate that the studied markers are involved in the pathogenesis of stage 3-4 CKD which will pave the way for novel prophylactic or therapeutic interventions since inflammation is a significant contributor to mortality in CKD patients.Finally, we recommend further studies to decipher the contribution of TLR-2 and other innate immune receptors in the regulation of inflammation, immune responses and injury in the kidney and to elucidate factors triggering TLR-2 expression. This will increase the importance of this signaling system as a potential therapeutic target. We also recommend repeating this study in stage 1-2 CKD patients aiming to solve the problems of early diagnosis in the asymptomatic period of the disease.
References
| [1] | Longmore M, Wilkinson IB, Davidson EH, Foulkes A and Mafi AR. Oxford Handbook of Clinical Medicine, Oxford University Press, Oxford, UK, 8th edition, 2010. |
| [2] | Steenkamp R, Castledine C, Feest T and Fogarty D. “UK Renal Registry 13th Annual Report (: Chapter 2: UK RRT prevalence in 2009: national and centre-specific analyses,” Nephron, vol. 119, no. 2, pp. 27–52, 2011. |
| [3] | Dungey M, Hull KL, Smith AC, Burton JO and Bishop NC. Inflammatory factors and exercise in chronic kidney disease. International Journal of Endocrinology. 2013; 569831-569839. |
| [4] | Arslan F, Keogh B, McGuirk P and Parker AE. TLR2 and TLR4 in ischemia reperfusion injury. Mediators of Inflammation. 2010; 704202-704210. |
| [5] | Koc M, Toprak A, Arikan H., Odabasi Z., Elbir Y., Tulunany A., Asicioglu E., Eksioglu-Demiralp E., Glorieux G., Vanholder R. and Akoglu E. Toll-like receptr expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant. 2011; 26: 955–963. |
| [6] | Meuwese C.L., Snaedal S. and Halbesma N. Trimestral variation of C-reactive protein, interleukin-6 and tumour necrosis factor-α are similarly associated with survival in haemodialysis patients,” Nephrology Dialysis Transplantation. 2011;26 (4): 1313–1318. |
| [7] | McMahon GM and Waikar SS. Biomarkers in Nephrology. Am J Kidney Dis. 2013; 62(1): 165–178. |
| [8] | Meldrum KK, Zhang H and Meldrum DR. Profibrotic effect of interleukin-18 in HK-2 cells is dependent on stimulation of the Toll-like Receptor 4 (TLR4) promoter and increased TLR45 expression. The Journal of Biological Chemistry 2012; 287(48):40391-40399. |
| [9] | Edelstein CL. Biomarkers in kidney disease. 1. Amsterdam; Boston: Academic Press/Elsevier; 2011. |
| [10] | Kumar PJ and Clark ML: Kumar and Clark’s Clinical Medicine, Saunders Elsevier, Edinburgh, UK, 7th edition, 2009. |
| [11] | Barreto DV, Barreto FC, Liabeuf S, et al. “Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney International, 2010; 77(6): 550–556. |
| [12] | Cheung WW, Paik KH and Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatric Nephrology. 2010; 25(4): 711–724. |
| [13] | Stenvinkel P, Ketteler M, Johnson RJ et al.: “IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia–the good, the bad, and the ugly,” Kidney International. 2005;67 (4):1216–1233. |
| [14] | Sirota JC, Klawitter J and Edelstein CL. Biomarkers of Acute Kidney Injury Journal of Toxicology. 2011; 328120-328130. |
| [15] | Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012 |
| [16] | Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: A review. Kidney Int. 2011; 80(8):806–821. |
| [17] | Dinarello CA, Novick D, Kim S and Kaplanski G. Interleukin-18 and IL-18 binding protein. Frontiers in immunology. 2013. |
| [18] | Rhee AC, Cain AL, Hile KL, Zhang H, Matsui F and Meldrum KK. IL-18 Activation is Dependent on Toll Like Receptor 4 during Renal Obstruction. J Surg Res. 2013; 183(1): 278–284. |
| [19] | Zubowska M, Wyka K, Fendler W, Mbynarski W and Zalewska-Szewczyk B. Interleukin 18 as a Marker of Chronic Nephropathy in Children after Anticancer Treatment Disease Markers. 2013; 35 (6): 811–818. |
| [20] | Kelly DS. Toll-like receptors in kidney disease Curr Opin Nephrol Hypertens. 2009; 18(3): 189–196. |
| [21] | Ortega O, Rodriguez I, Gallar P, et al. “Significance of high C-reactive protein levels in pre-dialysis patients,” Nephrology Dialysis Transplantation.2002; 17(6): 1105–1109. |
| [22] | Oberg BP, McMenamin E, Lucas FL et al. “Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease,” Kidney International. 2004; 65(3). 1009–1016. |
| [23] | Devaraj S, Jialal I, Yun J and Bremer A. Demonstration of Increased TLR2 and TLR4 Expression in Monocytes of Type 1 Diabetic Patients with Microvascular Complications. Metabolism 2011; 60(2): 256–259. |










 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

