-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(5): 99-105
doi:10.5923/j.cmd.20140405.04
Introduction and Implementation of Non-Microscopic Method for Malaria Diagnosis Using OptiMAL IT into the Blood Donation Routine Test in Aseer Region, Saudi Arabia
Saad M. Bin Dajem1, Essam H. Ibrahim1, 2, Osama M. S. Mostafa1, 3, Ali Alshehri1, Mona Kilany1, 4, Ali A. Aljeamelani1, 5, Hala F. Hadish6, 7, Yasser A. Zahar8, Abdulaziz A. Heijan6
1Department of Biology, Faculty of Science, King Khalid University, Abha, Saudi Arabia
2Department of Blood Products Quality Control and Research, National Organization for Research and Control of Biologicals, Cairo, Egypt
3Department of Zoology, Faculty of Science, Ain Shams University, Abbassia, Cairo, Egypt
4Department of Microbiology, National Organization for Drug Control and Research (NODCAR), Cairo, Egypt
5Biology Department, Faculty of Science and Education, Albaidah University, Albaidah, Yemen
6Blood Bank, Aseer Central Hospital, Abha, Saudi Arabia
7Department of Hematology, Teshreen Hospital, Damascus, Syria
8Blood Bank, Abha General Hospital, Abha, Saudi Arabia
Correspondence to: Essam H. Ibrahim, Department of Biology, Faculty of Science, King Khalid University, Abha, Saudi Arabia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Background: Rapid diagnostic tests for malaria are now a commonly used procedure for malaria diagnosis. Despite some problems related to sensitivity and applicability, malaria rapid diagnostic tests (RDTs), are currently considered the best option to overcome well trained experts in blood banks and fast releas of blood units. Objectives: To detect malarial parasites using OptiMAL IT rapid test and to compare this method with the peripheral blood smear (PBS) and polymerase chain reaction (PCR) methods. Methods: Blood samples were collected from 100 patients clinically confirmed of having malaria and from 6698 random healthy blood donor volunteers. These samples were used to perform PBS examination, the OptiMAL test and PCR by standard protocols. Results: PBS examination found malarial parasites in 100 (100%) patients samples with a parasite load more than 0.01% and negative for all samples of blood donor volunteers. Positive samples obtained in PBS were also positive by OptiMAL test without differentiating between mixed infection. PCR could detect P. falciparum in 100 (100%) patients samples and two (2%) were positive for P. vivax in addition to P. falciparum. Conclusions: OptiMAL IT rapid diagnostic test can replace the peripheral blood smear method in blood banks with taking into consideration the limit of kit parasite load detectability. PCR is the most sensitive method that can detect low parasitaemia and mixed infection.
Keywords: Malaria, OptiMAL IT rapid test, Peripheral blood smear method (PBS), PCR, Blood bank, Saudi Arabia
Cite this paper: Saad M. Bin Dajem, Essam H. Ibrahim, Osama M. S. Mostafa, Ali Alshehri, Mona Kilany, Ali A. Aljeamelani, Hala F. Hadish, Yasser A. Zahar, Abdulaziz A. Heijan, Introduction and Implementation of Non-Microscopic Method for Malaria Diagnosis Using OptiMAL IT into the Blood Donation Routine Test in Aseer Region, Saudi Arabia, Clinical Medicine and Diagnostics, Vol. 4 No. 5, 2014, pp. 99-105. doi: 10.5923/j.cmd.20140405.04.
Article Outline
1. Introduction
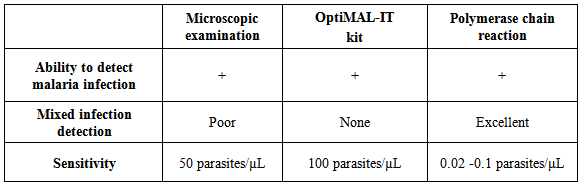
- Malaria continues to be a serious public health problem that cause high levels of morbidity and mortality in malaria-endemic regions. In 2012, there were an estimated 207 million cases of malaria and about 627,000 deaths [1]. The combination of high disease prevalence and high frequency of international travel creates a significant risk for the exportation of malaria to countries where the disease is nonendemic. This risk is accompanied by the possibility of introduction of malaria-causing organisms into the blood supplies used for transfusions. It was reported that the four principal species of Plasmodium that can infect humans have been transmitted via blood transfusion in the United States [2], Switzerland [3], France [4] and the United Kingdom [5]. This has resulted in the implementation of donor deferral policies in many countries that restrict blood donation by those with a history of recent travel to or from regions known to be malaria endemic and by those with recent cases of clinical malaria. The effectiveness of donor deferral programs has previously been questioned [6], and there is concern that many donors are unnecessarily deferred, since the rates of imported malaria are much lower than the rates of travel to endemic areas [7, 8]. To prevent unnecessarily reduction of qualified donor populations, some countries have implemented malaria antibody screening such that only individuals who are known to have been exposed to organisms causing malaria are subject to deferral of donations rather than all donors who have traveled to or lived in regions where malaria is endemic. Commercial antibody enzyme-linked immunosorbent assays (ELISAs) are currently in use in some countries like the United Kingdom, France, and Australia, and reinstatement of questionnaire- deferred donors was discussed in Canada and the United States [9-11]. In these cases, blood donors are tested for antibodies directed against Plasmodium-derived antigens within several months of deferral; when the tested individuals show negative antibody results, donation is allowed.The stained peripheral blood smears (PBS) examination by light microscopy is widely used to detect malarial parasites and this method remains the gold standard for the diagnosis of malaria. The conventional PBS examination remains the gold standard for malaria diagnosis at point of care level, and is commonly used to detect malarial parasites. However, it is laborious, time-consuming and its reading needs expertise, its accuracy and effectiveness may be unsatisfactory when performed in blood banks [12], its sensitivity and specificity are limited to the number of tests that can be analyzed per microscopist and his/her training, especially for low-parasite densities [13, 14]. In the context of the limitations of pure clinical diagnosis and the microscopic methods, especially in blood banks, there is a need for a rapid, prompt, specific and easy-to-perform test for the diagnosis of malariais essential for malaria control strategy. Tests that depends on parasite antigen detection have been introduced which target the parasite lactate dehydrogenase (pLDH) enzyme, present only in live parasites leading to high sensitivity and specificity. Also, the polymerase chain reaction (PCR) parasite nucleic acid detection test has been widely used to increase the sensitivity of malaria diagnosis, especially in the case of mixed infection and low parasitaemia. Transfusion transmitted malaria (TTM) especially in malaria endemic countries can be a major problem because those who are semi-immune individuals with low level of parasitemia remain asymptomatic and may be qualified as blood donors. Malaria parasites have the ability to survive in the storage conditions (4°C) of donated blood. The sensitivity of malaria screening by microscopic examination limit is about 50 parasites/µL and the rapid diagnostic device (RDT) limit is about 100 parasites/µL, these limits are far away of the required detection level of parasitemia that is capable of causing TTM which is about 0.00004 parasites/uL or 1-10 parasites/unit of blood [15].OptiMAL is a malarial antigen capture assay which detects Plasmodium-specific pLDH enzyme, a marker protein for the intraerythrocytic forms of the living malaria parasites. The test uses one monoclonal antibodies directed against epitopes of pLDH from P. falciparum and other monoclonal antibodies directed against the four species of Plasmodium spp. that infect human (P. falciparum, P. vivax, P. ovale and P. malariae). Anti-pLDH monoclonal antibodies used in the test allow the differentiation between Plasmodium falciparum and non-Plasmodium falciparum infections where one specific for P. falciparum and the other two are pan-specific for all [16]. Levels of pLDH in the blood correlate with the viability of the parasites and during treatment both peripheral parasitaemia and pLDH levels fall in response to effective chemotherapy [17].The routine serological tests according to predefined protocol of blood banking safety requirements by Saudi Ministry of Health comprised HBsAg, anti-HBc antibodies (Abs), anti-HCV-Abs, anti-HIV-1/2-Abs, anti-HTLV-I/II- Abs, Malaria, and Treponema Abs, as well as, nucleic acid test (NAT) technology for HCV-RNA, HBV-DNA, and HIV-RNA [18].In view of the above, this study was conducted to evaluate the possible introduction of rapid test using OptiMAL kit in blood banks found in Saudi Arabia as an alternative for the microscopic examination of PBS to ease and accelerate the release of blood units.
2. Materials and Methods
2.1. Subjects
- The study included 6698 random healthy blood donor volunteers, who were referred to blood transfusion centers found at Aseer region (Southern part of KSA) and signed informed consent, during the period from March 2012 to January 2013. According to routine practice, volunteer blood donors were interviewed and medically examined before donation. Those with high risk behaviors or those with any medical problem especially jaundice or hospitalization at fever hospitals, bleeding disorders necessitating component transfusion, pregnancy, or recent delivery less than 12 weeks were rejected.In addition, blood from 100 patients who were clinically and microscopically confirmed of having malaria from Aseer and Jazan provinces were included as a positive controls in this study after signing of informed consent.
2.2. Preparation of Blood Films, Spots and Microscopic Examination
- Thick and thin blood films were prepared and three drops from each participant were blotted onto 3-mm Whatman® filter paper (Whatman International Ltd., Maidstone, England). The filter papers were carefully air-dried at room temperature and stored under sealed conditions at 4°C until additional processing. The blood-spot samples were placed in plastic bags and transported to the laboratory. Blood films were stained with diluted Giemsa stain and examined microscopically for the presence of circulated malaria parasites using a 100× objective [19]. An examination of 15min for a thin blood film and 200 fields for a thick blood film were performed by two expert microscopists. Parasitaemia was estimated on all slides containing Plasmodia by counting 1000 red blood cells and expressing the number of parasitized cells seen as a percentage. For the purpose of this study, 1 µ1 of blood was considered to be equivalent to 5 × 106 red blood cells.
2.3. Detection of Malaria parasite Using PCR Method and Detection of Parasite Lactate Dehydrogenase (pLDH) Enzyme in Blood Samples
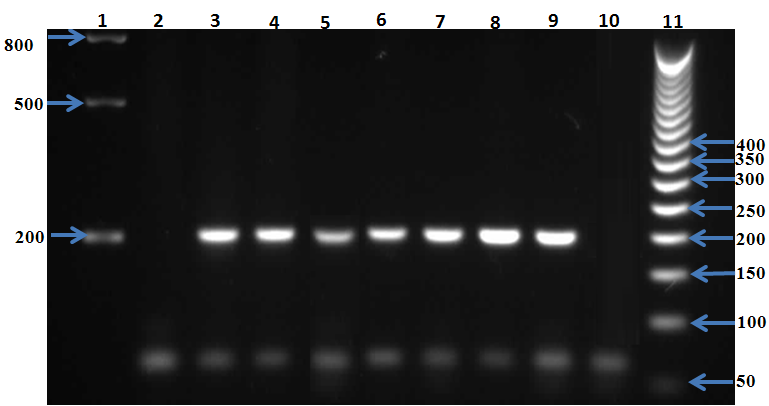
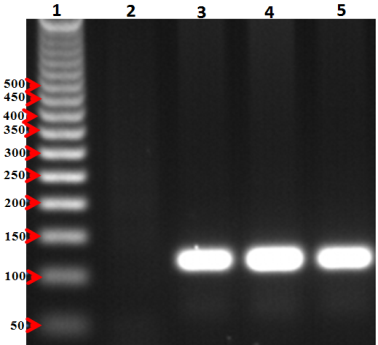
- For PCR assay, all blood samples (from in blood banks samples and known psitive malaria samples confirmed by the microscopic)were invitigated in this study. Genomic DNA was extracted from the all samples using a Qiagen kit (DNeasy® Blood & Tissue Kit, Hilden, Germany) according to the manufacturer’s instructions. Plasmodium species were also investigated by 18S rRNA-based nested PCR using genus- and species-specific primers as previously described [20, 21]. The products from the second round of nested PCR were analyzed by gel electrophoresis with a 1.5% (wlv) agarose gel. Pictures were taken using a gel documentation system (Quantum-Capt, VilberLourmat). Migration distances and band sizes were calculated.Detection of plasmodia in blood samples was done using the individual rapid malaria test OptiMAL-IT (BIO-RAD, France) for all samples. Optimal IT test is an immune-chromatographic test which use monoclonal antibodies against the metabolic enzymes parasite lactate dehydrogenase (pLDH) of Plasmodium spp. These monoclonal antibodies are lined into two lines in the test, one specific for Plasmodium falciparum and the other for pan-specific monoclonal antibodies which reacts with all 4 malaria species which infect human (P. falciarum, P.vivax, P. ovale and P. malariae). According to the manufacturer, the device can detect peripheral parasitaemia level of 0.001-0.002% (50-100 parasite per µL of blood). All samples were tested for malaria infection using Optimal IT test according to the manufacturer’s instructions. OptiMAL positive control (BIO-RAD), a recombinant pLDH was included in the test which gives a results exactly as patient infected with P. falciparum. Interpretation of the test results was done according to manufacturer instructions.
2.4. Statistical Analysis
- The biochemical data recorded were expressed as mean±SD and statistical and correlation analyses were undertaken using the one-way ANOVA followed by a post-hoc LSD (Least Significant Difference) test. A P value < 0.05 was statistically significant. A statistical analysis was performed with the Statistical Package for the Social Sciences for Windows (SPSS, version 10.0, Chicago, IL, USA).
3. Results
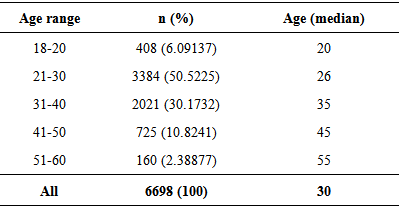
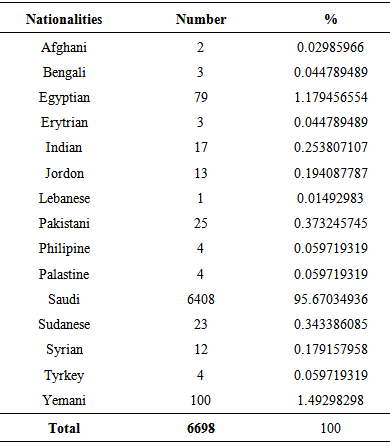
- The study was conducted on 6698 random healthy blood donor volunteers (19 females (0.28%) and 6679 males (99.72%)) with a median age of 28 and 30 years, respectively in addition to 100 malaria infected subjects. All donors showed normal blood pressure, pulse rate, hemoglobin level, and temperature and were voluntary non-remunerated blood donors and were qualified by a questionnaire authorized by Saudi MOH. Donors of ages between 21 and 30 years constituted the largest proportion (50.52%, P ≤ 0.001) with a median age of 26 years (Table 1). Table 2 lists the distribution of the nationalities of the study participants. Blood donors were mostly Saudi nationals (95.67%). The higher non-Saudi proportion was Yemenis (1.49%), followed by Egyptians (1.17%), Pakistani (0.37%), Sudanese (0.34%), Indians (0.25%), Jordanians (0.19%) and then Syrian (0.18%).
|
|
|
4. Discussion
- Malaria, the disease which is caused by protozoan parasites of the genus Plasmodium. Historically, strategies for malaria diagnosis have ranged from basic empirical clinical diagnostic means to the examination of stained peripheral blood smears by light microscope. Although empirical clinical diagnosis of malaria remains the most common method of diagnosis in several parts of the world, its accuracy is reduced as the symptoms of malaria may overlap with several other tropical infections where other tropical diseases such as dengue fever mimic each other’s symptoms (e.g., fever, chills, and headache). Precise diagnosis of malaria is important to prevent mortality and morbidity and avoiding unnecessary use of antimalarial drugs. This can avoid over-use of anti-malarial drugs, therefore delay the development of drug resistance and can also reduce the risk of adverse drug reactions due to unnecessary treatment and direct increase correct treatment for pathologies other than malariasis [22, 23].The conventional PBS examination is widely used in detection of malarial parasites and remains the gold standard for malaria diagnosis. But, it is laborious, time-consuming and its interpretation requires expertise [12]. In view of these limitations of clear and accurate clinical diagnosis using the microscopic methods, there is a need for a specific, simple-to-perform and rapid test for the diagnosis of malaria. Malaria antigen detection tests have been introduced which detect the presence of malaria lactate dehydrogenase enzyme, which present only in live parasites, and these offer high sensitivity and specificity. In addition, the polymerase chain reaction that detect genomes of the parasites has been widely used to increase the sensitivity of malaria diagnosis, especially in the case of low parasitaemia. As blood banks in Saudi Arabia receive considerable number of blood donors which in turn take much time and big efforts to perform PBS examination for malaria in addition to routine tests, OptiMAL –IT kit was indented to replace PBS to ease and accelerate the release of blood units.A total of 6698 blood donors were enrolled in the study. Majority of the subjects were Saudis, the rest of the subjects had many different nationalities that provided information on travel to malaria-endemic areas. In our study, the possibility of using Optimal-IT in blood banking was evaluated. OptiMAL-IT method was found to be simple, sensitive and effective diagnostic test for the diagnosis of malaria. It was found to be rapid diagnostic test and the final results could be read in 20 minutes. The sensitivity of this test was very close to that obtained by microscopic examination of PBS, and it did not require highly skilled personnel to perform or interpret its results. The test added the advantage of being able to detect all four Plasmodium species through detecting the pLDH enzyme produced only by living parasites. It is reasonably priced and no special facility is required for its storage. The major disadvantage of OptiMAL is its inability to differentiate between P. falciparum and mixed infection which has no bad feedback in blood banking. Several authors have previously reported trials of OptiMAL for the diagnosis of malaria with some disparity of comparative data. These findings are similar to some studies as some authors reported that more than 90% specificity and sensitivity of Optimal-IT [24, 25]. Tarimo et al. (2001) [25] even reported that Optimal-IT accuracy was almost ideal.Although microscopy is considered as the gold standard for diagnosis of malaria at point of care level, its accuracy may be satisfactory when performed in blood banks. This suggests an urgent need for capacity building of microscopists and/or the implementation of accurate rapid tests such as OptiMAL on a large scale. For our opinion we would prefer OptiMAL to replace PBS in blood bank which may be in accordance to Shillcutt et al. (2008) [26] who prefer RDT to microscopy. In our study, as confirmed by PCR, there were two cases of mixed P. falciparum/P. vivax. OptiMAL gave the results as P. falciparum. This could be expected from the configuration of the test where P. falciparum could react with both monoclonal antibodies on the strip and this might potentially mask another species if there was a mixed infection. The use of PCR in accurate detecting and identifying of Plasmodium species was useful but on the other hand it was time-consuming, requiring 3-5 hours to complete the test. In addition this test required high expertise, expensive infrastructure and its consumables and reagents are with high cost and required storage at -20°C [27]. False results may be obtained when using the microscopic diagnosis of the endemic populations especially in case of low parasitemia. It was suggested by Zaman et al. (2001) [28] that the main disadvantage of the microscopic diagnosis is the inability of diagnosing of the mixed infections and when only ring forms are seen. In addition, it was reported by Postigo et al. (1998) [29] that false-negative results by microscopic examination of P. vivax are probably due to very low parasitemia which is very difficult to detect by this mean. So in the time that conventional microscopy is considered to be the reference method and the one most commonly used for the diagnosis of Plasmodium spp, its specificity and sensitivity are essentially limited to the number of repeated tests that can be done per individual analyst and his/her training, especially in the case of low parasitaemia and subsequently more time is needed for an accurate diagnosis [13, 14]. These constrains may explain the false results that could be obtained in the microscopic diagnosis of the endemic populations. Previously, several workers have reported trials of OptiMAL kit for the diagnosis of malaria with some divergences of comparative data. In a study done by Jelinek et al. (1999) [30] on non-immune travelers, a number of patients were found to infected with P. falciparum by microscopy or PCR. Using rapid diagnostic kits, the authors showed sensitivity and specificity of 92.5% and 98.3% respectively for the HRP2 detection kit and a sensitivity and a specificity of 88.5% and 99.4% respectively for pLDH. In another study done by Palmer et al (1998) [31] where they microscopically examined positive blood samples for P. vivax and P. falciparum and found a sensitivity and specificity of 94% and 100% respectively for P. vivax and sensitivity and specificity of 88% and 99% respectively for P. falciparum using OptiMAL. Using some kits available that measure HRP2, a sensitivity of 65% was obtained for P. falciparum. On the other hand, in a retrospective trial done by John et al (1998) [32] in Southern India a sensitivity of 94% for P. falciparum and 98.2% for P. vivax were obtained. In another study done in the Gambia for initial diagnosis, Cooke et al (1999) [33] obtained a sensitivity and specificity of 91.3% and 94% respectively for P. falciparum with OptiMAL.On contrary, the findings obtained in our study were not consistent with some studies as a low sensitivity (ranging from 79 to 94%) and specificities (ranging from 97 to 100%) of the OptiMAL diagnostic test was shown by several reports from Afghanistan, Honduras, Kuwait, Turkey, and Peru [31, 34-37]. Other studies from Canada [38] showed a sensitivity of 29 but a specificity of 95.6%. In study done in Congo, the sensitivity of OptiMAL did not reach the acceptable threshold of 90% whereas the specificity was 97%.
5. Conclusions
- Screening blood donation for malaria infection using microscopy still the gold standard method. However, this method is laborious, time consuming and needs experts to perform. Molecular techniques are the most sensitive and accurate methods that can detect low parasitaemia and mixed infection, but also laborious, coasty and needs experts to perform. OptiMAL-IT rapid diagnostic test can replace the peripheral blood smear method in blood banks with taking into consideration the limit of kit parasite load delectability.
ACKNOWLEDGMENTS
- This research was supported by the King Khalid University, Project no. KKU-SCI-11-069. The authors gratefully acknowledge the contribution of Laboratory Managers and the staff members of the blood bank departments at Abha General Hospital and Aseer General Hospital.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML