-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(5): 90-98
doi:10.5923/j.cmd.20140405.03
Galectin-3 as Early Detector of Heart Failure in Children with Congenital Acyanotic Heart Disease
Layla A. Mohammed1, Heba S. Gafar2, Neama R. Hussien3
1Department of Cardiology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Pediatrics, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3Department of Clinical pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Heba S. Gafar, Department of Pediatrics, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
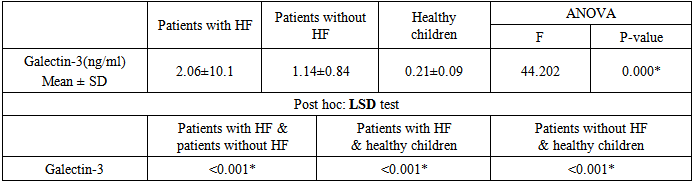
BACKGROUND:Heart failure (HF) is a common disease with complex pathophysiological causes. Diagnosis, risk assessment and treatment of HF are difficult and therefore there is a need for additional tools to improve clinical performance. Galectin-3 is a biomarker of inflammation and fibrosis, which is strongly associated with adverse remodeling and ventricular dysfunction. AIM: To evaluate the value of galectin-3 for prediction of HF among children with congenital heart diseases with left to right shunt. METHODS:Sixty congenital heart disease (CHD) children with left to right shunt were included. Thirty of them have heart failure (Ross II, III) and 30 patients without HF. In addition to 30 age and sex matched healthy children as a control group. Serum levels of galectin-3 in all cases and control subjects were determined. We investigated the correlation between galectin-3, Ross functional classification and echocardiographic finding. RESULTS:Levels of galectin-3 were 2.06±1.01 ng/ml & 1.14±0.84 ng/ml in patients with and without HF respectively, and 0.21±0.09 ng/ml in the control group. Galectin-3 was significantly higher in patients developed HF than those without HF (p<0.001). CONCLUSIONS:Galectin-3 is a promising novel cardiac biomarker which could reflect the progression of ventricular remodeling and allow early identification of HF.
Keywords: HF, Galectin-3, CHD
Cite this paper: Layla A. Mohammed, Heba S. Gafar, Neama R. Hussien, Galectin-3 as Early Detector of Heart Failure in Children with Congenital Acyanotic Heart Disease, Clinical Medicine and Diagnostics, Vol. 4 No. 5, 2014, pp. 90-98. doi: 10.5923/j.cmd.20140405.03.
1. Introduction
- Heart failure (HF) is a complex clinical syndrome characterized by ventricular dysfunction, neuroendocrine activation and abnormal peripheral blood flow distribution. Heart failure in congenital heart diseases with left-to right shunts is a serious problem encountered in early infancy or later; depending on the size of the shunt. It is a significant cause of morbidity and mortality in children with congenital heart disease [1].The diagnosis of HF commonly relies on comprehensive analyses of medical history and symptoms, and results of echocardiography and biochemical tests [2]. Circulating biomarkers that directly reflect disease progression, hemodynamics, and ventricular remodeling at a molecular level are critical for risk stratification in HF, affording unique insights into pathophysiology not fully captured by traditional risk markers, reduce the cost effectiveness and improve the treatment outcomes of HF patients [3].Galectin-3 is a soluble β-galactoside-binding lectin that has been shown to mediate fibrosis in a variety of organ systems including the heart [4]. Galectin-3 is nearly undetectable in cardiomyocytes, but in responce to myocardial insult, Galectin-3 is released by activated cardiac macrophages and induces fibroblasts proliferation and depositation of type I collagen in the myocardium through activating transforming growth factor β (TGFβ) and stimulating matrix production [5, 6].There is growing evidence that galectin-3 is directly involved in the pathophysiology of cardiac injury and progression to HF, making it a potential diagnostic, prognostic and therapeutic target [7].
2. Subjects and Methods
- A prospective comparative study was conducted on 60 children having CHD with left to right shunt. They were selected from Al-Zahraa university hospital (pediatric and cardiology departments) and National heart institute (Cairo, Egypt) between January 2014 and September 2014. Thirty of them have manifestation of HF, and 30 without HF; their age ranged from 6-36 months. Additionally, 30 age and sex matched healthy children were set as a control group. All subjects were informed of the purpose of the study and parent consent was obtained.Exclusion criteriaChildren with cyanotic heart disease, valvular lesions, cardiomyopathy or undergo previous surgical correction. Also those with non-cardiac systemic chronic diseases were excluded.Clinical history and examinationAll patients were subjected to a full clinical history and examination performed by pediatrician and cardiologist. The relevant clinical history includes the demographic data, presenting symptomes, any previous surgry, and current medications. The findings of systemic and cardiac examinations were recorded in details. Assessment of growth by anthropometric measurements was represented by weight and length/height, which were plotted on Egyptian growth Charts 2002 [8] to detect weight for age and height/length for age percentiles. Body surface area was determined by blotting of weight and length on the surface area nomogram [9].Identification of HFDiagnosis of HF was based on clinical evaluation, modified Ross scoring (II-IV), X-ray and echocardiographic examination. The modified Ross Classification incorporates feeding difficulties, growth problems, and symptoms of exercise intolerance into a numeric score comparable with the New York Heart Association (NYHA) classification for adults which is not applicable to most of pediatric population [10]. The Ross Classification was developed to provide a global assessment of HF severity in infants, and has subsequently been modified to apply to all pediatric ages [11].Echocardiography examinationA complete echocardiographic examination was performed for all subjects in both supine and left lateral position using VIVID 7 GE systems. All cases were examined using multiple transducers ranging from 3.5 to 7MHZ. with simultaneous electrocardiographic recording to allow timing of flow. Uncooperative children were given chloral hydrate according to their body weight (50- 75mg/kg).M-Mode Measurements:M-mode was obtained from LV parasternal short axis view by applying cursor line perpendicular at midpapillary level to measure the following: Left ventricular internal dimensions at the end systole and diastole (LVESD, LVEDD) respectively, fractional shortening (FS%), ejection fraction (EF%) and left atrium diastolic dimension. FS % varies with age (ranges from 35-45%) and EF% normal value is approximately 55-65% in children [12].Two dimensional echo (2D echo):2D echo images were obtained from the parasternal (long and short axis), subcostal and apical views (apical four and five chambers) to assess the anatomy and prove the diagnosis of different types of CHD, to show anatomy and integrity of atrioventricular valves. Doppler echocardiography: Pulsed wave Doppler of the mitral and tricuspid valves flow was obtained from apical four and five chamber views with sample volume placed at the tip of the mitral and tricuspid valves, the transmitral and tricuspid inflow velocity were traced and early diastolic (E), Late diastolic (A) velocities and E/A ratio were measured. Continuous Doppler study of the Pulmonary and aortic valves were performed to assess the systolic pressure gradient across the pulmonary and aortic valves.Measurement of galectin-3 levelFive ml of venous blood samples were withdrawn from each subject, serum samples were obtained after clotting and centrifugation of blood at 3000 rpm for 5 min., the sera obtained were divided into 2 tubes, one was stored at −70°C for galectin-3 analyses and the second was analyzed for renal function. Another sample was taken for blood count.Serum Galectin-3 level was determined by using commercially obtained immunoassay, Quantitative Sandwich enzyme linked immmunosorbent assay (ELISA) technique, Golry Science Co., Ltd ; 2400 veternas BLvd. Del Rio, Tx 78840, USA.A double antibody Sandwich ELISA was used to measure the level of galectine-3. The assay system utilizes one galectin-3 antibody for solid phase (microtiter wells) immobilization and another galectin-3 antibody in the antibody-enzyme (horseradish peroxidase) conjugate solution. The test specimen (serum) is added to the galectin-3 coated microtiter wells and incubated. If galectin-3 is present in the specimen, it will combine with the antibody on the well. The well is then washed to remove any residual test specimen, and galectin-3 antibody labeled with biotine and combined with streptavidine-HRS is added to form immune complex; then carry out incubation and washing again to remove the uncombined enzyme. The conjugate will bind immunologically to the galectin-3 on the well, resulting in the galectin-3 molecules being sandwiched between the solid phase and enzyme-linked antibodies, then the chromogen solution A & B is added, resulting in the development of a blue color. And at the effect of acid, the color is finally become yellow and measured spectrophotometrically at 450 nm. The concentration of galectin-3 is directly proportional to the color intensity of the test sample.Statistical analysesData were analyzed using Statistical Package for Social Science (SPSS) version 16. Demographic & clinical data were presented as mean values ± standard deviations or as percentages. Weight and height were expressed in terms of standard deviation score (Z-score). Difference between two groups was compared by t-test for parametric data and differences among three groups were compared by one-way analysis of variance (ANOVA) test. Pearson’s correlation coefficient was used to determine correlations between Galectin-3 and different variables. Receive Operation Curve (ROC) analysis was carried out to identify the optimal cut-off points of the Galectin-3 concentration for prediction of HF. P value < 0.05 was considered significant.
3. Results
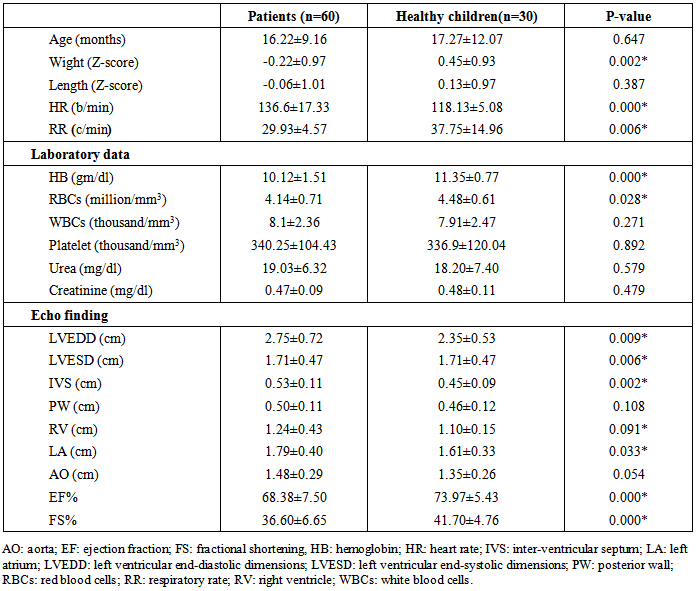
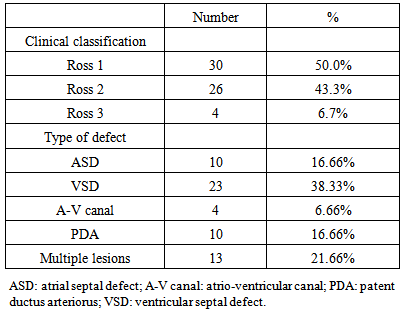
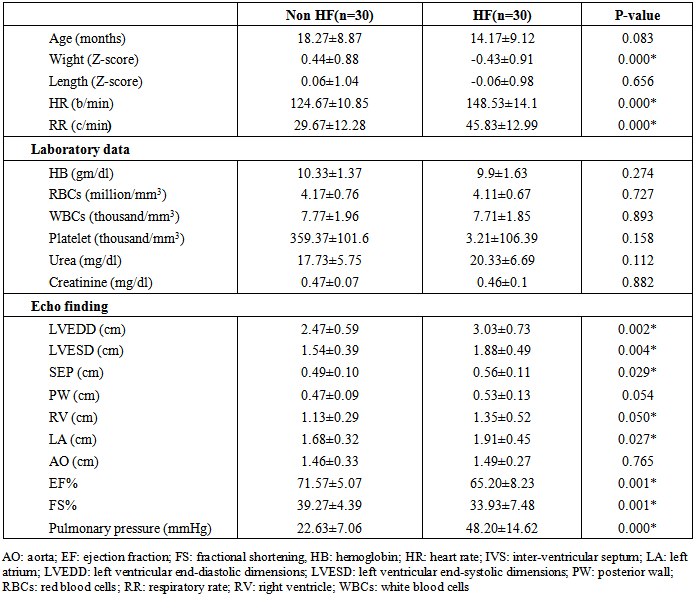
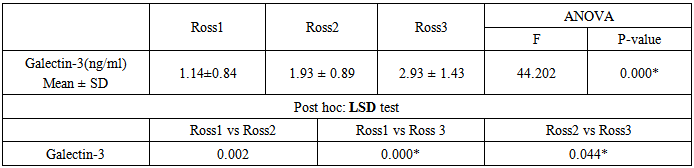
- General characteristics of the study patient population The study included 60 children had CHD with left to right shunt (28 males and 32 females). Their age ranged between 5-36 months. In addition to 30 healthy children as a control group (14 male, 16 females); their age ranged between 6-42 months. Table 1 shows the general characteristics, laboratory investigations, and echocardiographic findings of the children included in the study. No substantial age and gender differences were found among children involved in the study. Children with CHD had significant evidence of growth impairement for both wight and length for age (Z-score P-value < 0.05). Nutritional affection was reflected by significant lower level of Hemoglobin among cardiac children (P <0.005).
|
|
|
|
|
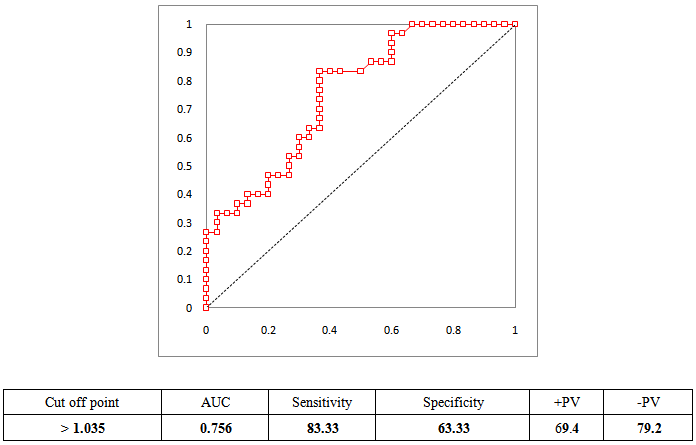 | Figure 1. Sensitivity, specificity, and predictive values of HF diagnosis by galectin-3 level |
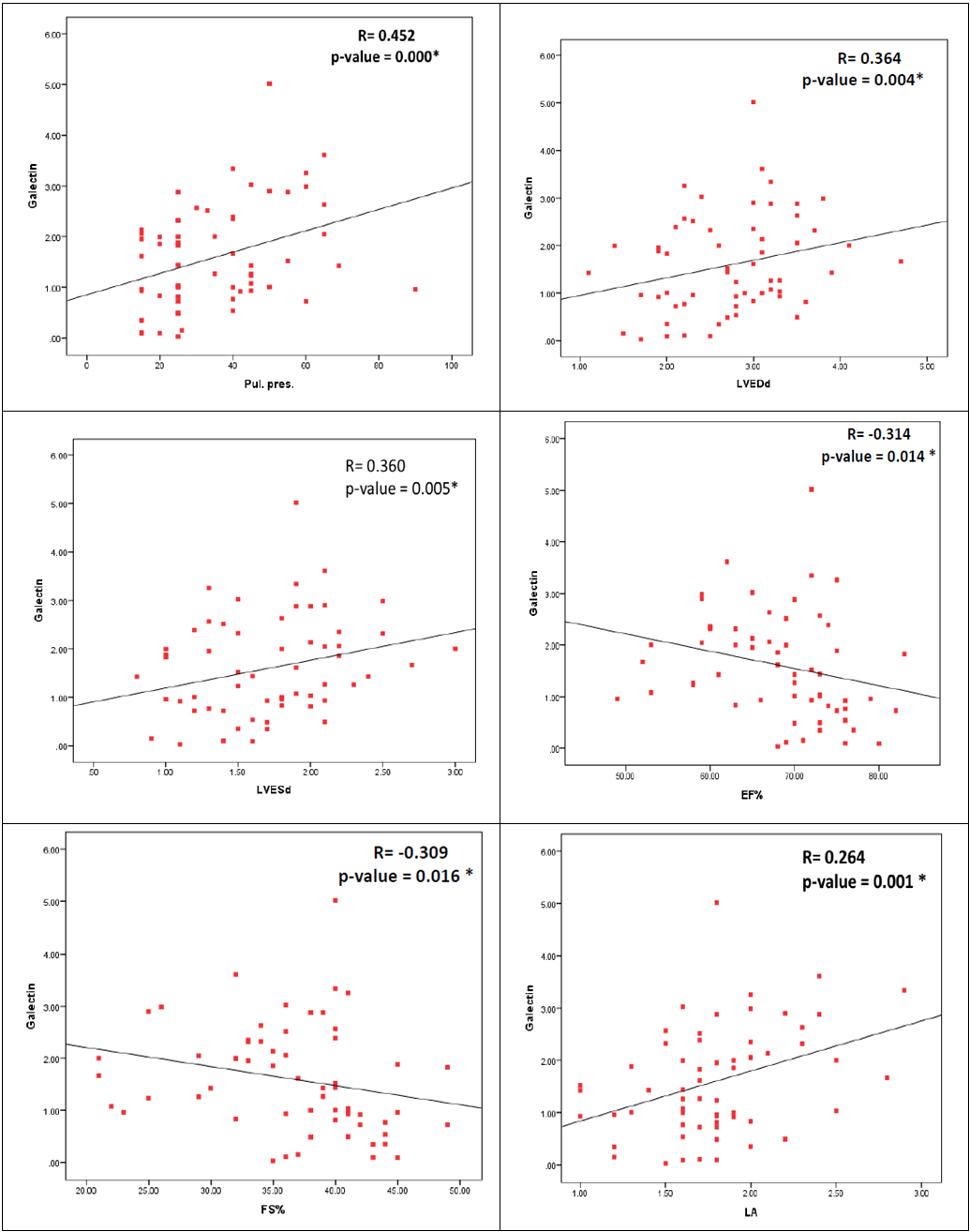 | Figure 2. Correlations between serum galactin-3 level and echo finding in cardiac patients |
4. Discussion
- The development of HF is often a clinically silent process, with progressive cardiac remodeling that eventually leads to symptomatic presentation late in the course of disease progression [13]. Using cardiac biomarkers may be one avenue in which patients may be routinely evaluated for the presence and severity of HF [14].Galectin-3 plays an important regulatory role in cardiac fibrosis and remodeling, which are key contributing mechanisms to the development and progression of HF [15, 16]. In adult population, normal serum concentrations of galectin-3 are <11ng/ml [17]. There is limited data about serum galectin-3 level in children, in clinical studies serum galectin-3 in neonate ranged between 0–1.8 ng/ml [18], while in older children its level ranged between 0.0 and 2.14 ng/ml (median 0.73 ng/ml) [19].In rat models, galectin-3 expression was found to be up-regulated in myocardial biopsies of rats with cardiac hypertrophy that subsequently progressed to HF and administration of galectin-3 leads to progressive fibrosis and LV systolic dysfunction [20]. These findings were confirmed in human myocardial biopsies, with higher levels of galectin-3 in those with poorer LVEFs [21]. In spite of the growing evidence of the use of serum galectin-3 in adult for diagnosis and risk stratification of HF, there is limited data about the utility of galectin-3 as cardiac marker in children. From our best knowledge, this study was the first to investigate the role of galectin-3 in assessing the risk of development of HF in children with CHD.In adult studies, galectin-3 levels were higher in HF patients versus controls, and higher galectin-3 levels were associated with measures of HF severity, including higher NYHA classification, worse treatment outcome [22, 23], longer hospitalization and greater mortality [24, 25]. This relationship is presented in both HF with reduced or preserved EF% [26] and even in acute HF [27]. Additionally, elevated galectin-3 could predict cardiovascular adverse events and incident HF in healthy populations [28-30].To investigate the role of galectin-3 as a marker of ventricular remodeling in children with CHD either with or without HF, we found that serum galectin-3 level was significantly higher in children with CHD than age and sex matched healthy children (p<0.001). Additionally, there was significant evidence of cardiac remodeling and significantly worsen EF detected by echocardiography between cardiac patients and healthy children. This finding demonstrates the role of galectin-3 as a marker of cardiac remodeling not only in adult but also in children.Expression of galectin-3 appears to occur before evident HF and thus may be more useful for prediction and prevention of disease sequel [31]. In our study, galectin-3 level was significantly elevated in children with HF in comparison to those without HF, with significant correlation with the disease severity, including higher Ross classification and echocardiographic finding even in those with normal EF.In children significant left-to-right shunting can cause congestive HF symptoms despite normal systolic ventricular function [32]. This could explain why most of our cases have preserved ejection fraction > 50% even those with HF. Jaarsma et al, 2008[33] had concluded that Galectin-3 may be a stronger prognostic marker in those with preserved compared with reduced EF. Gopal and colleagues, 2012 [34] had demonstrated that Galectin-3 levels were similarly elevated in all patients with HF, regardless of whether it was acute or chronic or it was systolic or diastolic in nature.In our study, there was significant positive correlation between serum galectin-3 level and left atrial & ventricular dimensions and pulmonary pressure, with significant negative correlation with LVEF. In adult, elevated galectin-3 was associated with echocardiographic parameters representative of impaired cardiac function and unfavorable remodeling [35].The clinical and cardiac structural correlations with galectin-3 that explored in our study provide the first indirect evidence supporting a potential role for galectin-3 in the pathogenesis of cardiac remodeling in CHD children with left to right shunt who developed HF. These observations demonstrate that galectin-3 can be used not only as a potential HF biomarker that could reflect ongoing ventricular remodeling but also as an early risk stratification marker in children with CHD. Contradictory to our results, higher cut off point of galectin-3 were reported by adult studies. Chen et al., 2013 [36] demonstrated that galectin-3 level 7.52 ng/ml has 62.9% sensitivity and 90% specificity to predict congestive heart failure (CHF). While levels ≥17.8 ng/mL was predictive of rehospitalization and mortality [37]. Increased levels of galectin-3 are related to increasing age, progressive renal dysfunction and severity of HF [38]. Additionaly, galectin-3 expression is modulated by genetic variences [39]. These data could explain the previous conflicting results. Further studies at larger scale of patients are required to reach an agreed prognostic cut-off value for galectin-3 in children with HF.An advantage of using galectin-3 for assessing cardiovascular risk is that galectin-3 levels tend to be more stable over time with less intraindividual variability as compared with natriuretic peptides or C-reactive protein [40]. Additionally, unlike galectin-3, the previous cardiac biomarkers are generally elevated as a result of the disease process and do not substantively contribute to disease progression [41].Although, Natriuretic peptides, are widely used to guide the management of HF patients, they only indicate ventricular loading condition and do not reveal other important mechanisms in HF [42]. Galectin-3 may signal different aspects of pathophysiologic processes in HF than BNP so galectin-3, as a novel biomarker for HF, could provide additional information in the diagnosis and prognosis of HF.Moreover, pharmacologic inhibition of galectin-3 has been shown to attenuate cardiac fibrosis, remodeling and prevent the development of HF in animal studies [43]. Taken together, these data suggest that galectin-3 may be a potential pathway that could be targeted pharmacologically, to alter the development of HF [44].Although significant work is still required to further define the role of galectin-3, it has the potential to become an important part of future heart failure management algorithms by helping to provide an individualized risk profile, which can be used to optimize resource allocation and improve treatment outcomes [44].
5. Conclusions
- Galectin-3 is promising cardiac biomarker for HF diagnosis and risk stratification in children with CHD. Serum galectin-3 level is elevated in HF patients and its level was related to HF severity, including Ross classification and echocardiographic finding. In CHD children, galectin-3 level could reflect the progression of ventricular remodeling and allow early identification of these changes before the progression to overt HF. Currently, there are several commercially available assays that can measure circulating Galectin-3, if validated; it will be a powerful tool in identifying risk and simplifying the diagnosis of a condition that currently requires costly investigations, subspecialist interpretations and early management.Further researches confirming the structural correlations observed in this study, as well as examining the impact of therapeutic intervention on galectin-3 levels are needed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML