-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(5): 83-89
doi:10.5923/j.cmd.20140405.02
Anti-glomerular Basement Membrane Antibodies and Cystatin C as a Predictive Markers of Renal Dysfunction in Chronic Hepatitis C Virus Patients
Ashraf T. Abd Elmouttaleb1, Ebrahim M. Bayomy2, Abdelraouf A. Abonar2, Mohamed S. Eldeen Radwan2, Gamal M. Soliman3
1Medical Biochemistry Department, Assisted Reproductive Unit, International Islamic Center for Population studies and research, Al-Azhar University, Egypt
2Clinical Pathology Department, Faculty of Medicine, Al-Azhar University, Egypt
3Tropical Medicine Department, Faculty of Medicine, Al-Azhar University, Egypt
Correspondence to: Ashraf T. Abd Elmouttaleb, Medical Biochemistry Department, Assisted Reproductive Unit, International Islamic Center for Population studies and research, Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
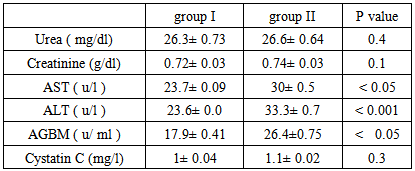
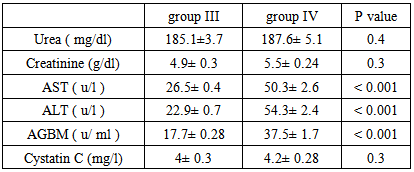
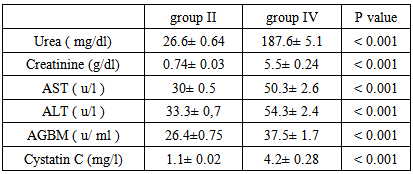
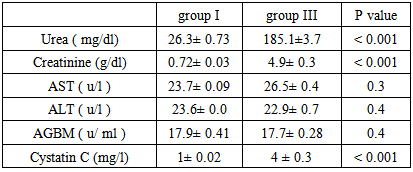
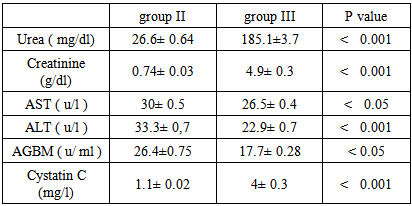
Background and study aim: There is an increasing recognition of association between hepatitis virus infection and glomerular diseases, renal complication may be the presenting manifestation of HCV infection. One of the most important recent laboratory parameters as diagnostic marker for autoimmune renal disease is the Anti-Glomerular Basement Membrane (Anit-GBM) antibodies. In recent years, serum cystatin C has been proposed as a marker of early kidney dysfunction. The main aim of this study is to detect the role of Anti-GBM antibodies and cystatin C as a predictive markers of early renal impairment in HCV infected patients. Patients and methods: This study was conducted on 75 patients (30-60 years old) and 20 completely healthy persons at the same age. The studied subjects were divided into the following groups: Group I: 20 Control group of completely healthy persons. Group II: 25 Positive HCV with normal renal functions. Group III: 25 Negative HCV with renal failure on hemodialysis. Group IV: 25 Positive HCV with renal failure on hemodialysis. All patients and control groups were selected by ELISA test for diagnosis of HCV infection, HCV positive groups are confirmed by PCR test, complete renal and liver functions were performed. Anti-GBM (Anti Glomerular Basement Membrane antibodies) and cystatin C were assayed by ELISA technique. Results: There is a high significant increase in Anti-GBM antibodies in group II and group IV patients compared with control group G I (P < 0.001). Also there is a high significant increase in AGBM antibodies in group II and group IV patients more than group III (P < 0.001). There is no significant difference in Anti-GBM antibodies between group III and group I, which means that the source of Anti-GBM is HCV infection. There is a high significant increase of cystatin C in group III and group IV patients compared with group I or group II (P < 0.001), but there is no significant difference between Group II and Group I which means that the elevated cystatin C level is related to renal disease such as renal failure in this study and not affected by HCV infection. There are positive significant correlations between cystatin C and creatinine in all studied groups where (r = 47 and p = 0 .031), (r = 0.49 and p = 0.021), (r = 0.55 and p = 0.001), (r = 0.62 and p = 0.001) in groups I, II, III, IV, respectively. Conclusions:It is concluded that the serum anti glomerular basement membrane antibodies are a marker of HCV infection and they are increased in those with HCV infection and renal failure requiring hemodialysis while serum cystatin C level could be used as a predictive marker of renal dysfunction whether associated with HCV infection or not.
Keywords: HCV, Cystatin C, Anti GBM-antibodies
Cite this paper: Ashraf T. Abd Elmouttaleb, Ebrahim M. Bayomy, Abdelraouf A. Abonar, Mohamed S. Eldeen Radwan, Gamal M. Soliman, Anti-glomerular Basement Membrane Antibodies and Cystatin C as a Predictive Markers of Renal Dysfunction in Chronic Hepatitis C Virus Patients, Clinical Medicine and Diagnostics, Vol. 4 No. 5, 2014, pp. 83-89. doi: 10.5923/j.cmd.20140405.02.
Article Outline
1. Introduction
- Hepatitis C Virus (HCV) is a hepatotropic virus and a common cause of chronic hepatitis. It can cause a variety of extrahepatic immunological manifestations [1]. There is an increasing recognition of association between hepatitis virus infection and glomerular diseases [2]. Renal complication may be the presenting manifestation of HCV infection [3]. Some authors detected HCV core proteins with membranous glomerulonephritis [4].The most common cause of virus-associated nephropathies is hepatitis virus, especially HBV or HCV Combes et al [5], described the first case of membranous nephropathy with HBV infection in 1971. Johnson et al [6] first introduced eight patients with MPGN and HCV infection in 1993. In contrast, the histomorphological pattern of glomerulonephritis associated with viral hepatitis A has not been well recognized, because hepatitis A is relatively rare and a self-limited disease without chronic progression.Crook et al [7] reported that HCV as a predictor of poorer renal survival in diabetic patients. HCV chronic liver disease can be associated with plethora of immune and autoimmune perturbations and many authors claim that HCV chronic infection can play an important role of the pathogenesis of these disorders [8].One of the most important recent laboratory parameters as diagnostic marker for autoimmune renal disease is the Anti-Glomerular Basement Membrane (Anit-GBM) antibodies. These autoantibodies directed against type IV collagen in the glomerular basement membrane. Binding of these antibodies to the basement membrane induce rapidly progressive glomerulonephritis. First, it was discovered to be associated with renal disease of various systemic autoimmune disorders, eg lupus erythematosus (SLE), vasculitis syndromes and infection process [9]. Then its significance was also reported in early diagnosis of lupus nephropathy also as a predictive marker of sub-clinical, post-streptococcal glomerulonephritis [10].Cystatin C is a cationic non-glycosylated protein, formed from 120 amino acids. It is produced at a constant rate in lysosomes of all nucleated cells of the body, and is present in diverse biological fluids like serum, semen and cerebrospinal fluid. It is cleared exclusively through the kidney and therefore its concentration depends solely on the GFR [11].Cystatin C is a potent inhibitor of cysteine protease, and it is found in extracellular fluids. It is a low molecular weight protein (13.359 kDa). Clearance of cystatin C cannot be measured because it is broken down in the cells of the proximal tubule, whereas its serum concentration is a good marker of GFR, has been identified as a promising marker of renal failure. Recent studies have shown that serum cystatin C can be used as an accurate marker of GFR in adults and children [12].Cystatin C determinations have been reported for several diseases and altered cystatin C concentrations have been detected in patients with autoimmune disease, amyloidosis and multiple sclerosis [13]. Cystatin C has been detected in carcinoma cell lines, and alterations in the balance between endogenous inhibitors and cysteine proteinases have also been associated with malignant tumor progression [14].Clinically, cystatin C has been applied as a sensitive marker of glomerular filtration rate and an early indicator of impaired renal function and has been proved to be superior to serum creatinine. The determination of cystatin C serum concentration appears to be independent of sex, age, muscle mass, hyperbilirubinemia or haemolysis, and diet has no significant impact on serum cystatin C concentrations. Furthermore, the intra-individual variation of cystatin C in healthy controls has been shown to be extremely low [15].The main aim of this study is to detect the role of Anti-GBM antibodies and cystatin C as a predictive markers of early renal impairment in HCV infected patients.
2. Patients and Methods
- This study was conducted on 75 patients with same age (30-60 years old), selected from the outpatient clinic of Tropical Medicine at Al Hussein University Hospital, and 20 completely healthy persons as a control group at the same age. Hepatitis C virus positive patients were infected by HCV nearly 4-9 years before this study. Cases were divided as detected by ELISA, PCR and kidney function tests into the following groups:• GI: Control group completely healthy persons (20 person).• GII: Positive HCV with normal renal functions (25 cases).• GIII: Negative HCV with renal failure on hemodialysis (25 cases).• GIV: Positive HCV with renal failure on hemodialysis (25 cases). Patients of Group IV are on hemodialysis 2-4 years before HCV infection.Exclusion criteria:1. Patients with diabetes, hypertension or systemic lupus.2. Any cause of liver disease other than HCV based on patient history clinical or laboratory findings e.g.a. Autoimmune hepatitis.b. Wilson disease.c. Drug induced liver disease.d. Alcoholic liver disease.e. Hbs Ag positive patient3. Patients with advanced liver disease such as decompensated liver disease (portal hypertension or hepatosplenomegaly), cirrhotic, nodular, enlarged or coarse livers are excluded. Hepatic tumors are also excluded by α fetoprotein, abdominal ultrasonography or CT scan.All patient groups are clinically, ultrasonography and laboratory without advanced liver disease.All cases were subjected to assay of serum creatinine, blood urea, AST and ALT. Renal and liver function tests were determined by using Hitachi (912) apparatus with commercial kits of ROCH company. HCV antibodies were detected by 3rd generation ELISA (Enzyme-Linked Immunosobent Assay) kits (Diasorin-Italy) and confirmed by conventional PCR (Cobas Ampliprep/Cobas Taqman roche Diagnostics). Anti-GBM antibodies were detected by ELISA technique according to Larry, 2001 using commercial kit of DRG instrument Gmblt, Germany Drogen-identification. Cystatin C was measured by ELISA kit (Enzyme-Linked Immunosobent Assay) by RayBiotech, Inc. USA.Abdominal ultrasonography was done for all control and patient groups, all selected patients and control groups have smooth liver by ultrasonography.
3. Statistical Analysis
- Results were statistically analyzed with statistical package for social science (SPSS version 13). All data are expressed as mean ± SD and student t-test was used for comparison between studied groups. Significant result is considered if P <0.05. Highly significant result is considered if P <0.001. Spearman correlation coefficient test was performed for study of regression analysis.
4. Results
- This study was conducted on 75 patients with same age (30-60 years old), the study included both male (45) and female (30). The base line viral loads after PCR confirmation for patients with positive ELISA were:1. < 0.4 x 106 IU/ml = 32 patients = 64% (low viraemia).2. > 0.4 x 106 IU/ml = 18 patients = 34% (high viraemia).Our study shows results of Anti glomerular basement membrane (AGBM) antibodies, serum Cystatin C, kidney and liver function (urea, creatinine, AST and ALT).
|
|
|
|
|
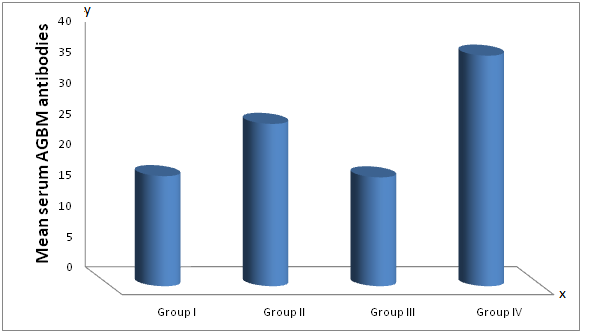 | Figure (1). Mean serum anti glomerular basement membrane (AGBM) antibodies in different studied groups |
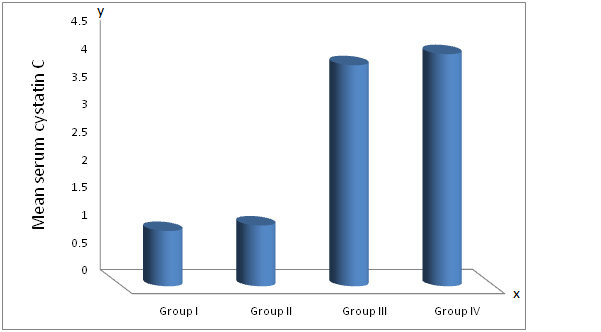 | Figure (2). Mean serum Cystatin C in different studied groups |
5. Discussion
- Extrahepatic syndromes have been reported in as much as 36% of HCV patients, but the exact prevalence is not known. The most prevalent extrahepatic disease with the highest degree of association with HCV are the essential mixed cryoglobulins with skin, neurologic, renal, and rheumatologic complications. Non cryoglobulin diseases with a less definite relationship to HCV include systemic vasculitis, splenic lymphoma, porphyria cutanea tarda and sicca syndromes [16]. Diabetes, thyroid disease, and presence of autoantibodies in the serum are also linked to HCV but less strongly. The pathophysiologic basis for most of these syndromes seems immunologic. Cirrhosis and chronic HCV infection seem to be risk factors [17].The extrahepatic manifestations that share mild degree certainty of association with HCV infection include B-cell non-Hodgkin lymphoma, autoimmune thrombocytopenia, pruritis and type II diabetes. The mechanisms through which HCV may promote or induce extrahepatic manifestations remain unclear [18].HCV can cause membranoproliferativeGlomerulonephritis (MPGN), membranous glomerulonephritis, cryoglobunemic glomerulonephritis and also IgA nephropathy. HCV infection is a significant cause of MPGN especially in countries where HCV is highly prevalent [19].HCV is a lymphotropic virus, in addition to its being hepatotropic. This peculair lymphotropism may be responsible at least in part for the multiple immune mediated extrahepatic manifestations of HCV infection such as mixed cryoglobinemia, the presence of rheumatoid factor (RF) in serum and the production of autoantibodies [20].HCV virus has the theoretical potential to cause renal injury by a number of direct or indirect mechanisms. Circulating immune complexes of anti-HCV antibodies and viral components, often augmented by anti-immunoglobulin rheumatoid factors, have been detected in numerous studies; such complexes may lodge in the renal glomerulus and cause damage. It also has also been shown to elicit autoimmune responses against several "self" antigens, in some cases by molecular mimicry; some of the resulting autoantibodies have known or suspected associations with renal disease. Hepatic injury caused by HCV has a variety of systemic consequences, including decreased clearance of circulating immune complexes, decreased synthetic function for numerous serum proteins, and hemodynamic perturbations, with known or potential consequences for the kidney [21].Renal disease occurs in about half of patients with mixed cryoglobinaemia associated with HCV infection. These patients present with purpura ,arthralgia, neuropathy and abdominal pain secondary to mesentric vasculitis. Such symptoms of mixed cryoglobinaemia often manifest years before a diagnosis of renal disease is made. Acute nephritic syndrome usually is concomitant with acute flare up of systemic signs of mixed cryoglobinaemia. Massive precipitation of cryoglobulins in the glomerular capillary lumen with consequent severe monocytic infiltration often with signs of systemic and renal vasculitis is responsible for this syndrome [4].This study showed that Anti-GBM antibodies are highly significantly increased in patients of Group II (positive for HCV with normal renal function) and in patients of group IV (positive for HCV with renal failure on haemodialysis) compared to group I (negative for HCV with normal renal function). Moreover there is a high significant increase in Anti-GBM antibodies in patients of group IV, (positive for HCV with renal failure on hemodialysis) compared with group II (positive for HCV with normal renal function). There is no significant difference in anti GBM antibodies between patients of group III and group I. These results indicate that Anti-GBM antibodies have strong role of kidney pathology and are related to HCV infection.These results demonstrated that the source of Anti-GBM is HCV infection goes hand in hand with many studies which indicated a major role of chronic HCV infection in immune mediated diseases such as membranoproliferative Glomerulonephritis (MPGN) in the study of Parakash et al [22], and the study of Me Guire et al [23] they demonstrated that, immune -complex glomerulonephritis is common in patients with HCV induced cirrhosis and is often clinically silent in these patients.In this study, the mean cystatin C level is significantly elevated in groups III and IV compared to groups I and II, respectively, the mean cystatin C level is (4, 4.2 mg/l) in groups III and IV, respectively and it is (1, 1.2 mg/l) in groups I and II, respectively (P < 0.001). The mean cystatin C level is non significantly elevated in groups II and IV compared to groups I or group III, respectively (p = 0.3). These results indicate that the elevated cystatin C level is related to renal disease such as renal failure and the elevated serum cystain C level is an indicator of renal impairment whether associated with HCV infection or not.This is in agreement with the study of Cahide et al [24] they found that plasma cystatin C is a more sensitive marker of glomerular filtration rate than creatinine in adults with chronic renal failure and suggested that plasma cystatin C value may be used as a marker of glomerular function at the laboratory diagnosis of chronic renal failure, the same results were founded in children by Raul narvaez et al [25] they reported that the serum cystatin C is a very interesting option , and could be a replacement to serum creatinine for diagnosis and possibly for monitoring kidney function in children.In this study the measurement of serum cystatin C in liver disease (HCV infection) has no value in detection of early renal impairment in HCV infected patients, but in another study its measurement could be useful as in its measurement in advanced liver cirrhosis as reported in the study of Maha barakat and Mohamed khalil [26] for detection of early renal dysfunction in advanced liver cirrhosis, they described that early hepatorenal syndrome can be diagnosed by the elevated serum cystatin C level in advanced cirrhotic patients having normal serum creatinine.This study does not agree with the study of Jarsozewcz et al [27] they found that the mean serum cystatin C level is significantly elevated in chronic hepatitis C patients compared to control group and asymptomatic impairment of renal function may be detected in a proportion of chronic hepatitis C patients by use of cystatin C but not creatinine based GFR –estimated. This discrepancy may be due to different studied population or different stage of liver disease as demonstrated by Shu Chen et al [28] they has revealed that a direct relation between serum cystatin C with severity and progression of chronic liver disease.In this study serum cystatin C was found to show positive significant correlation with serum creatinine in all studied groups which concedes with the previous studies of Cahide et al [24], Le Bricon et al [29] and Plebani et al [30].On the other hand, we have found a positive non significant correlations between cystatin C and viral loads in HCV infected patients which do not agree with the results of the study of Behairy et al [31] they reported inverse correlation between cystatin C viral load in children but no other studies were done in adults.
6. Conclusions
- It is concluded serum cystatin C level could be used as a predictive marker for renal dysfunction whether associated with HCV infection or not. It is possible that AGBM antibodies can be used as marker of HCV associated nephritis, but this cannot be clearly determined based on this study approach. Future studies should be done on a large number of population with HCV infection with follow up of AGBM antibodies over a long time specially that eventually developed renal failure is recommended.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML