-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(4): 65-70
doi:10.5923/j.cmd.20140404.02
Effect of Late Luteal Phase Clomiphene Citrate on Ovulation Induction and Endometrial Blood Flow in Patients with Polycystic Ovary Syndrome
Samia Mohamad Eid1, Mostafa Mohammad Shakweer2
1Department of Obstetrics & Gynecology, Faculty of Medicine, Al-Azhar University, Damietta, Egypt
2Department of Radio Diagnosis, Faculty of Medicine, Al-Azhar University, Damietta, Egypt
Correspondence to: Samia Mohamad Eid, Department of Obstetrics & Gynecology, Faculty of Medicine, Al-Azhar University, Damietta, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
BACKGROUND: Clomiphene citrate (CC) is the most commonly used drug for treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS), have shown a discrepancy between ovulation and pregnancy rates. This may be partly explained by the peripheral antiestrogenic effects at the level of the endometrium and cervical mucus, by hypersecretion of LH, or due to negative effects of CC on oocytes or granulosa cells (GC). It can be argued that these negative effects are augmented by the relatively long half-life of CC which is known to be 5 days. If treatment is started late in the cycle preceding the induced cycle, those negative effects are more likely to extend into the sensitive peri-implantation period. In the present study we compare the effect of late luteal phase CC administration to early follicular phase day 3 on induction of ovulation and several ultrasonographic markers of uterine receptivity in PCOS patients. METHODS: The present study is designed as a prospective clinical study. Two hundred (200) infertile women aged 20-35 years old with the diagnosis of PCOS with oligo-hypomenorrhea were be examined at Faculty of Medicine Al-Azhar University, Damietta in department of Obstetrics and Gynecology at the Infertility Outpatient Clinic. Group I (study group, late luteal phase CC): included 100 PCO patients to whom 100 mg of clomiphene citrate were administered daily for five days starting the next day after finishing medroxyprogesterone acetate (MPA) 10 mg tablets for 5 days. Group II (control group, early follicular phase CC): included 100 PCO patients to whom 100 mg of clomiphene citrate were administered daily for five days starting in day 3 of withdrawal bleeding induced by medroxyprogesterone acetate (MPA) 10 mg tablets for 5 days. RESULTS: The proportion of women who had more than one mature follicle was significantly higher in women in study group when compared to women in control group (P=0.009).The mean endometrial thickness at day of human chorionic gonadotropin (hCG) administration was significantly higher among women in study group when compared to women in control group (P<0.001). The mean endometrial and subendometrial resistance index (RI) at day of hCG administration and midluteal was significantly lower (denoting higher subendometrial blood flow) among women in study group when compared to women in control group (P<0.05). CONCLUSIONS: Late luteal phase clomiphene citrate results in significant growth of more than one mature follicle and improve endometrial blood flow compared to early follicular phase CC.
Keywords: Polycystic ovary syndrome, Clomiphene citrate, Late luteal phase, Endometrial blood flow
Cite this paper: Samia Mohamad Eid, Mostafa Mohammad Shakweer, Effect of Late Luteal Phase Clomiphene Citrate on Ovulation Induction and Endometrial Blood Flow in Patients with Polycystic Ovary Syndrome, Clinical Medicine and Diagnostics, Vol. 4 No. 4, 2014, pp. 65-70. doi: 10.5923/j.cmd.20140404.02.
Article Outline
1. Introduction
- Polycystic ovary syndrome (PCOS) is a common heterogeneous endocrine disorder characterized by irregular menses, hyperandrogenism, and polycystic ovaries. The prevalence of PCOS varies depending on which criteria are used to make the diagnosis, but is as high as 15%-20% when the European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine criteria are used. Clinical manifestations include oligomenorrhea or amenorrhea, hirsutism, and frequently infertility [1].Clomiphene citrate (CC) constitutes one of the first line treatments for ovulation induction in PCOs patients since it is economical, has few adverse effects and requires little monitoring. CC is an estrogen antagonist that produces an increase in circulating follicle stimulating hormone (FSH) concentrations by negative feedback blockage, there by inducing follicular growth [2]. It induced a transient rise in LH and FSH during the treatment (CC peak), which was followed by a pattern of gonadotropin secretion closely resembling that observed in the normal ovulatory cycles [3].Although CC is very successful in induction of ovulation, there is usually a discrepancy between ovulation and pregnancy rates (PR). The notable features are an ovulation rate of 73%, a pregnancy rate of 36%, and a live birth rate of 29%. This may be partly explained by the peripheral antiestrogenic effects at the level of the endometrium and cervical mucus, by hypersecretion of luteinizing hormone (LH), or due to negative effects of CC on oocytes or granulosa cells [4]. Approximately 15% of women who take CC have poor postcoital test results, and intrauterine insemination (IUI) is recommended for these women [5].Evaluation of endometrial receptivity remains a challenge in clinical practice. Ultrasonography has an increasingly important role in the evaluation and treatment of infertile patients, being an efficient and cost effective modality for studying the female reproductive organs and for monitoring functional changes during spontaneous and induced cycles [6].The advent of transvaginal color Doppler sonography has added a new dimension to the diagnosis and treatment of infertile female. Color Doppler innovation is a unique noninvasive technology to investigate the circulation of organs, like uterus and ovaries [7].The endometrial blood flow reflects more properly the uterine receptivity because the endometrium is the place where the embryonic implantation is going to take place. The absence of the color map at the endometrial and subendometrial levels leads to a significantly decrease of the implantation rate whereas the pregnancy rate increases when vessels reach the subendometrial halo and the endometrium [7].In current study this question was tested, does starting CC in late luteal phase will improve the ovarian response and endometrial blood flow than early follicular phase clomiphene citrate? Therefore endometrial thickness and color Doppler assessment are applied to study of uterine and endometrial/subendometrial perfusion that is also used as receptivity factors.
2. Patients and Methods
- The present study is designed as a prospective clinical study. Two hundred (200) infertile women aged 20-35 years old with the diagnosis of PCOS with oligohypomenorrhea were be examined at Faculty of Medicine Al-Azhar University, Damietta in department of Obstetrics and Gynecology at the Infertility Outpatient Clinic. Those patients randomly allocated into two groups.Group I (study group): late luteal phase group which included 100 PCO patients to whom 100 mg of clomiphene citrate were administered daily for five days starting the next day after finishing medroxyprogesterone acetate (MPA) 10 mg tablets for 5 days.Group II (control group): early follicular phase group which included 100 PCO patients to whom 100 mg of clomiphene citrate were administered daily for five days starting in day 3 of withdrawal bleeding induced by medroxy- progesterone acetate (MPA) 10 mg tablets for 5 days.Women excluded in this study: Patient with regular cycle, age more than 35 years, major pelvic pathology, other infertility factors including male and tubal factor, ovarian masses, uterine or vaginal pathology that may interfere with Doppler assessment, patients with hepatic or renal impairment or hypersensitivity to clomiphene citrate that interfere with its administration, patient with hyper prolactinemia. All patients were monitored by transvaginal ultrasound in both transverse and Sagittal planes for: mean follicular volume, endometrial thickness (full-thickness measured from the myometrial-endometrial junction to the endometrial-myometrial junction) on days 10, 12, and 14 of the cycle, Endometrial layering, Myometrial contractions, Myometrial echogenicity, uterine artery Doppler flow and Endometrial blood flow evaluation. Serum estradiol (E2) (in picogram per milliliter) was measured at the time of chorionic gonadotropin (hCG) injection. hCG injection (5,000–10,000 IU IM) was given when at least one follicle measured 18 mm. Patients were advised to have regular intercourse every other day from day when any of growing follicle reach 16mm till 3days after HCG administration. Pregnancy test was determined 2 weeks after hCG injection in the absence of menstruation for diagnosis of pregnancy.The outcome measures were number of mature follicles, endometrial thickness, endometrial and subendometrial resistance index (RI) at day of hCG administration and midluteal phase.
3. Statistical Analysis
- Data were statistically described in terms of mean ± standard deviation (± SD), median and range, or frequencies (number of cases) and percentages when appropriate. Comparison of numerical variables between the study groups was done using Student t test for independent samples. For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. p values less than 0.05 was considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
4. Results
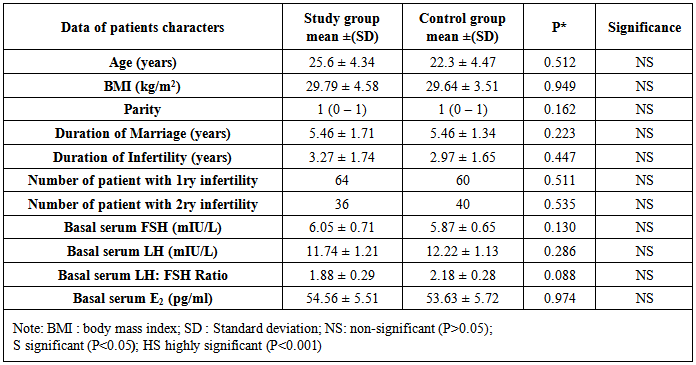
- The two groups show no difference between them as regards age, BMI, parity, duration of marriage, duration of infertility and number of patients with primary and secondary infertility. (Table 1)
|
|
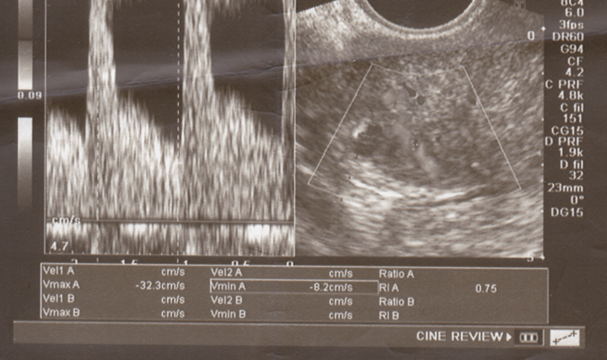 | Figure (1). Subendometrial RI (0.75) at day of hCG administration in a control group case |
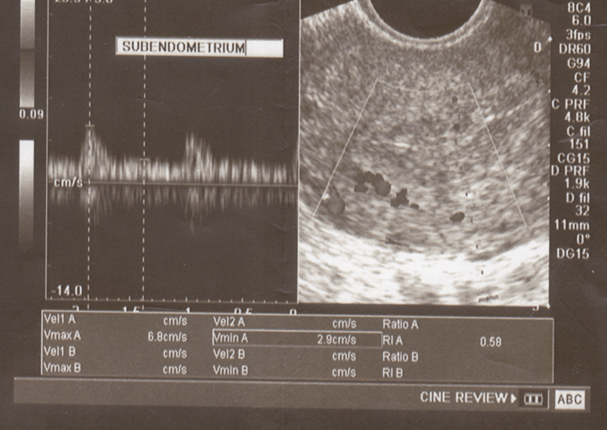 | Figure (2). Midluteal Subendometrial RI (0.58) in a study group case and trilaminar appearance of endometrium |
5. Discussion
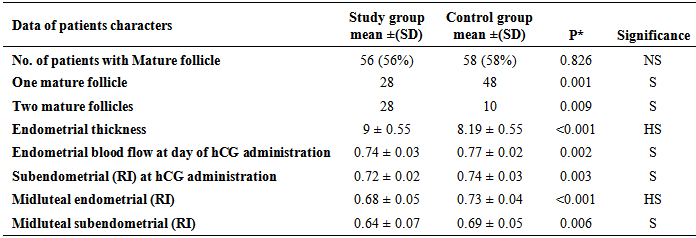
- Clomiphene citrate has an antiestrogenic effect on the hypothalamus, which induces ovulation, but its effect on other estrogen-sensitive tissues may be primarily agonistic or antagonistic. It is usually administered for a period of 5 days, starting on the fifth day of the menstrual cycle. Although CC induced ovulation rates are between 80% and 85%, conception rates are only around 40% [8]. This could be due to negative effects of CC on oocyte or granulosa cells, or because of prolonged antiestrogenic effects on the endometrium and cervical mucosa. These negative effects are augmented by the relatively long half-life of CC, which is known to be 5 days [5]. If treatment is started late in the cycle, those negative effects are more likely to extend into the sensitive peri-implantation period.In this study the proportion of women who ovulated (56%) was slightly lower among women in study group (late luteal phase CC) when compared to women in control group (early follicular phase CC) (58%); the difference was insignificant (P>0.05). However, the proportion of women who had two mature follicles ≥18 mm during stimulation was significantly higher in women in study group when compared to women in control group (P<0.05).This result was found to be in partial agreement with the results of Bilijan et al. [4] who found a more rapid follicular growth and a higher pregnancy rate when CC treatment was begun on day 1 rather than on day 5 of the cycle, probably due to larger recruitment of follicles as recruitment occurs early before the cycle. The ovulation rates of the present study are in agreement with the results of Rostami-Hodjegan et al. [9] who reported that 67% of women with PCO responded to the conventional administration of CC 100 mg per day with ovulation.When these results comparable with those of Badawy et al., 2009 [10] who reported an ovulation rate of 59.1% in the early CC group (luteal phase CC) compared to an ovulation rate of 51.9% in the late CC group (conventional CC administration), however, the difference was insignificant. Also they reported that the total number of follicles and the number of mature follicles ≥18 mm during stimulation were significantly more when CC was started in luteal phase. In our results the number of two or more mature follicles significantly increase with patient of late luteal phase CC, but the ovulation rate was higher in early follicular phase CC compared to late luteal phase CC (58% -56% respectively).Dominant follicle within the ovary is recognized by transvaginal color Doppler by day 8th or 10th of the cycle by a ring of angiogenesis around it, when compared to the subordinate follicles which do not demonstrate this. These vessels become more abundant and prominent as the follicle grows to about 20 to 24 mm in size [6].Infertility in women with polycystic ovary syndrome (PCOS) is not only attributed to anovulation but also to endometrial dysfunction [10]. Endometrium of the women with PCOS exhibited a lower number of glands and thicker luminal epithelium, in addition, reduced integrin compared to the normal women during the secretory phase [11]. It is also accepted that clomiphene citrate reduces uterine receptivity and thus the chances of conception [12].Several ultrasonographic parameters including the assessment of the endometrial thickness, endometrial and subendometrial blood vessels characteristics, were done in this study. The mean endometrial thickness at day of hCG administration was significantly higher among women in study group (9mm) when compared to women in control group (8.19mm) (P<0.001).This may be explained by the results of Bilijan et al. [4] who stated that if CC treatment is started early in the cycle, the negative effect of the drug on the endometrium (contributed to the relatively long half-life of CC) may be avoided.This is in consistence with the results of Badawy et al. [10] who reported that mean endometrial thickness at day of hCG administration was significantly higher among women in luteal phase CC (9.1mm) compared to women in conventional CC administration (8.2mm) (P<0.001).Similarly, the results of the current study are in partial agreement with those of Farhi and colleagues [13] who reported that the mean endometrial thickness at day of hCG administration was higher when CC was started at day 5 of menstrual cycle (7.1mm) than when CC was started at day ≥7 of menstrual cycle (6.8mm).An adequate uterine blood supply appears to be an important determinant of endometrial receptivity during natural conception and after assisted reproductive treatment. For quantitative purposes, the pulsatility index (PI) became widely used because of its accurate reflection of blood flow impedance and because it may be used when the end diastolic frequency shift is absent or reversed. The majority of studies have concentrated on measurement of blood flow within the uterine artery. Uterine artery PI is considered best at reflecting uterine artery blood velocity parameters and has been studied to evaluate uterine receptivity. However, it is still under discussion whether uterine artery PI could reflect uterine receptivity [14].The RI in the uterine artery hovers around 0.88 ± 0.04 until day 13 of the 28 days menstrual cycle. Increased uterine artery impedance is seen 3 days after the LH peak (day 16). This is explained by increased contractility and compression of vessels traversing the uterine wall, which decrease their diameter and consequently cause higher resistance to flow. Lowest blood flow impedance occurs during peak luteal phase (RI = 0.84 ± 0.04) during which implantation is likely to occur. RI of radial vessels is 0.78 ± 0.10 [15].This is the first study to our knowledge that investigate endometrial and subendometrial blood flow with late luteal phase administration of CC compared to early follicular phase as regards endometrial receptivity.In the present study, the mean endometrial resistance index (RI) at day of hCG was significantly lower (denoting higher sub-endometrial blood flow) among women in study group (0.74) when compared to women in control group (0.77) (P<0.05).Our data confirmed that the mean subendometrial RI at day of HCG administration was significantly lower (denoting higher blood flow) among women in study group (0.72) when compared to women in control group (0.74) (p<0.05).The results of the current study are in partial agreement with those of Cheung et al. [16] who reported that the mean subendometrial RI at day of hCG administration was 0.75 when CC was started at day 2 of the menstrual cycle and 0.72 when CC was started at day 5 of the menstrual cycle compared to 0.74 among non-stimulated cycles, denoting no statistically significant difference (P>0.05) in endometrial receptivity when CC was started on day 2 or on day 5.Also, the results of the present study are in consistence with those of Ng et al. [17] who concluded that endometrial and subendometrial blood flows were higher in natural cycles than in cycles stimulated by the conventional CC protocol as assessed by color Doppler.Chien et al. [18] showed, in fact, that the pregnancy and implantation rates were related to subendometrial flow and vascular penetration, whereas no relationship was detected with uterine artery impedance indices. In the present study, the midluteal endometrial resistance index (RI) was significantly lower (denoting higher endometrial blood flow) among women in study group (0.64) when compared to women in control group (0.54) (P<0.05). Also, the midluteal subendometrial RI was significantly lower (denoting higher subendometrial blood flow) among women in study group (0.64) when compared to women in control group (0.69) (P<0.05). By analyzing these results with previously reported studies it was found that it coincides with those of Palomba et al. [19] who found similar results where the midluteal endometrial resistance index (RI) was 0.63 in the conventional CC group (CC on day 3) compared to 0.55 in the control (non-stimulated) group, whereas the midluteal subendometrial RI was 0.57 in the conventional CC group (CC on day 3) compared to 0.53 in the control (non-stimulated) group. This denotes higher endometrial and sub-endometrial vascular impedance in the luteal phase of stimulated cycles when CC was started on day 3. This confirms the concept that the administration of clomiphene citrate in PCOS patients on day 3, even if effective for inducing ovulatory cycles in PCOS patients, worsens the subendometrial and endometrial vascularization.
6. Conclusions
- This study suggested that Late luteal phase administration of CC improve endometrial receptivity by lowering RI of both endometrium and subendometrium blood flow in patients having ooligo-hypomenorrhea due to PCOS. Also it was associated with more follicular growth, which might result in higher pregnancy rates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML