-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(4): 61-64
doi:10.5923/j.cmd.20140404.01
A Case of Resistant Hypertension Secondary to Primary Aldosteronism
Fathimah M.1, Gunavathy M.2, Nadzimah MN1
1Department of Pathology, Faculty of Medicine, Mara University Technology (UiTM), Sungai Buloh, 47000, Malaysia
2Department of Endocrinology, Internal Medicine Unit, Sungai Buloh Hospital, Sungai Buloh, 47000, Malaysia
Correspondence to: Fathimah M., Department of Pathology, Faculty of Medicine, Mara University Technology (UiTM), Sungai Buloh, 47000, Malaysia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Our patient is a 45-year-old lady with difficult to control hypertension despite on multiple antihypertensive. Her laboratory results showed persistent severe hypokalaemia with metabolic alkalosis. Further investigations revealed elevated aldosterone: rennin ratio (ARR) which suggested primary aldosteronism. Saline suppression test failed to suppress her plasma aldosterone level, which confirmed the diagnosis. A nodule which features compatible with lipid-rich adenoma was observed on computed tomography scan (CT scan). Left adrenalectomy succeeded to normalise her potassium level and keep her blood pressure under control with two antihypernsives.
Keywords: Resistant hypertension, Hypokalaemia, Primary aldosteronism
Cite this paper: Fathimah M., Gunavathy M., Nadzimah MN, A Case of Resistant Hypertension Secondary to Primary Aldosteronism, Clinical Medicine and Diagnostics, Vol. 4 No. 4, 2014, pp. 61-64. doi: 10.5923/j.cmd.20140404.01.
1. Introduction
- Primary aldosteronism (PA) was first described by Jerome W. Conn in 1954 [1]. It is a group of disorders in which the adrenals produce inappropriately high aldosterone which is relatively autonomous from the renin-angiotensin system. In primary aldosteronism, the aldosterone production will not be suppressible by sodium loading due to an excessive aldosterone secretion by the adrenal gland.Recent studies indicate that primary aldosteronism is a much more common cause of secondary hypertension than had been demonstrated historically. Cross-sectional and prospective studies report PA in more than 10% of hypertensive patients, both in general and in specialty settings [2, 3]. Most cases of PA belong to one of two subtypes: unilateral aldosterone-producing adenoma (APA) or bilateral adrenal hyperplasia (IHA), but some cases are classified into less common subtypes such as glucocorticoid responsive aldosteronism [4] and other familial forms [5]. The differential diagnosis between bilateral hyperplasia and unilateral adenoma however remains a medical challenge, and the pathogenic stimulus for hyperplasia is still unknown.PA plays an important role in cardiovascular diseases and should be systematically sought and specifically treated. This is because recent studies has revealed that patient with PA has higher cardiovascular morbidity and mortality than age-and-sex-matched patients with essential hypertension and the same degree of blood pressure elevation [6, 7]. These findings have precipitated a marked resurgence of research activity on this disorder as specific treatments are available that are able to ameliorate the impact of this condition on patient-important outcomes. We report here a case of a 45 year-old lady with resistant hypertension, severe hypokalaemia, and metabolic alkalosis who presented with PA caused by adrenal adenoma and was successfully treated by unilateral adrenalectomy.
2. Case Report
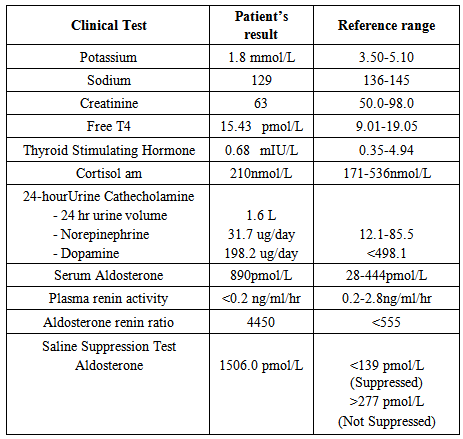
- A 45-year-old Malay lady initially presented to Sungai Buloh Hospital for menorrhagia secondary to a large uterine fibroid. During her admission to the gynaecological ward, they noticed that her blood pressure was difficult to control despite on multiple antihypertensives. Hence, she was referred to the medical team for further evaluation. She was first diagnosed to have hypertension eight years ago by a district health clinic at the age of 37 years old. Her blood pressure ranged from 160 to 180 (systolic pressure) and 110 to 130mmHg (diastolic pressure) despite on multiple antihypertensive drugs. Her medications include T. nifedipine 10mg three times a day, T hydrochlorothiazide 250mg daily, T captopril 37.5mg daily, T metoprolol 200 mg daily, and T prazosin 0.5 mg three times a day.She did not have any symptoms suggestive of phaeochromocytoma, Cushing’s syndrome, thyroid disorder or hyperparathyroidism. She denied having any headache, gastrointestinal disturbances, visual loss or any muscle weakness prior to this. There were no symptoms of renal disease and she never had any cardiac event before. She was otherwise well with no other comorbidities (eg: diabetes mellitus, dyslipidaemia, kidney disease, and etc). She had never smoked cigarettes nor consumed alcohol. None of her family members had adrenal or other endocrine tumors. Both of her parents have diabetes mellitus and hypertension which was diagnosed in the old age.Her physical examination revealed a blood pressure of 180/110 mmHg with no postural variation. Pulse rate was 80 per minute (regularly, regular) and she was not tachypnoiec with a respiratory rate of 18 per minute. She was not obese with a body mass index (BMI) of 19kg/m2. Fundi examination revealed Grade II retinopathy. Systemic examinations were unremarkable except that her apex beat was displaced to the left sixth intercostal space. There were no signs suggestive of coarctation of aorta.Blood investigation revealed a severe hypokalaemia with potassium of 1.8mmol/L. Her blood gas analysis showed mild metabolic alkalosis with pH of 7.5 and bicarbonate of 32 mmol/L. She was extensively investigated for secondary causes of hypertension. Her biochemical investigations were not suggestive of renal disease or any other endocrine disorders (phaeochromocytoma, Cushing’s syndrome, thyroid disorders, or hyperparathyroidism). Interestingly, her plasma aldosterone concentration was found to be elevated (890pmol/L) but her plasma renin activity was suppressed to <0.2ng/mL/hr. Therefore her aldosterone renin ratio (ARR) was markedly elevated, 4450 (normal : <555). The results of the laboratory investigations are summarized in Table 1.
|
3. Discussion
- Primary aldosteronism is common in patients with resistant hypertension with a prevalence of approximately 17-23% [8-11]. Numerous outcome studies have demonstrated that the high aldosterone state will lead to increased morbidity and mortality even when the blood pressure is controlled. [12-14]. Therefore an early diagnosis is very crucial in view of its prevalence and the fact that, this condition is treatable with surgical or medical therapy. Early diagnosis may help to prevent or reduce the complications attributed by this disease. Primary aldosteronism was first described by Jerome W. Conn [5] in 1955. He described this condition as characterized by hypertension, hypokalemia, suppressed plasma renin activity, and increased aldosterone secretion. In this case, this patient presented with almost the entire constellation of symptoms and metabolic effects described in aldosterone excess state. Unfortunately, there was a delay in a thorough investigation of PA even though her presentation is very classical for PA. She has been diagnosed with hypertension for the past eight years at the age of 37. She was not investigated as a young hypertensive for resistant hypertension and her diagnosis remained obscure for more than eight years. Her blood pressure throughout the years was poorly controlled despite on five different classes of antihypertensive drugs which should alert the clinicians of resistant hypertension. The diagnosis was finally unveiled itself when she was referred to the endocrine team during her hospitalization for unrelated symptom. It should be noted that the prevalence of primary aldosteronism has increased since the last decade. However recent prevalence studies revealed that PA is still under-diagnosed. It is often detected at the late manifestation. This is something that should be avoided in order to prevent or delay the unwanted complications. As for this patient, she has developed cardiomegaly as shown on the chest X-ray and electrocardiography (ECG).Resistant hypertension is defined as blood pressure that remains above target in spite of the concurrent use of 3 antihypertensive agents of different classes [8]. Interestingly she did not have any symptom despite a very severe hypokalaemia with potassium of 1.8mmol/L. According to previous studies, serum potassium levels were rarely low at the time of diagnosis of early primary aldosteronism, suggesting that hypokalemia is a late manifestation of the disorder preceded by the development of hypertension [15-17]. This is in accordance with this case. Therefore, hypokalaemia is not a necessary prerequisite for screening of the disorder, and perhaps all patients with hypertension should be screened for PA, given the known end-organ sequelae of excess aldosterone. The growing recognition of PA as a common and important contributor to the development of hypertension and cardiovascular disease has led to a resurgence in interest in the detection and diagnostic workup of this disorder by clinicians involved in the treatment of hypertensive patients. The ratio of plasma aldosterone concentration to plasma renin activity has been generally accepted as a first-line case-finding test. If a patient has an increased ratio, autonomous aldosterone production must be confirmed with an aldosterone suppression test. An increased aldosterone / renin ratio has 78% sensitivity and 83% specificity for PA, while the saline suppression test has 90% sensitivity and 84% specificity for hyperaldosteronism [18-20]. It is very crucial for the clinicians to adequately prepare the patient for the screening and confirmatory tests. This is to prevent false positive or false negative which may occur due to the lack preparation of the patient. Normokalaemia is essential as potassium level will cause interference in the renin-angiotensin-aldosterone system. Protocols must be provided and clinicians should be aware of the conditions or any drugs which may affect or influence the test results. For the confirmatory test, there are four different tests. It has been recommended by the Endocrinology Society that patients with a positive ARR measurement should continue with any of the four confirmatory tests in order to definitely confirmed or exclude the diagnosis. However there are some issues with regards to the confirmatory test. Each of the tests differs in terms of its sensitivity and specificity value. Furthermore, there is still no standardizing guideline on which cut off to use in each confirmatory test. In addition, currently there is insufficient direct evidence to recommend confirmatory test one over the others. Therefore the choice of the confirmatory test is commonly determined by the needs to consider the cost, patient compliance, laboratory routine and local expertise. In our setting, we commonly perform saline deprivation test. However caution must be given in patient with uncontrolled hypertension and congestive heart failure. Diagnostic work up for differential diagnosis of PA is controversial, generally non definitive and furthermore some of the methods may not be available at every medical centre [21]. The Endocrine Society has recommended AVS to be pursued in all patients who is a candidate for surgery [22]. This is to avoid the risk of an unnecessary unilateral adrenalectomy. However in this case, in view of the patient’s age, the imaging phenotype, and her refusal to proceed with the AVS, therefore surgery was done without prior AVS.Fortunately, the patient was well postoperatively and she only required two antihypertensives for her blood pressure control and her potassium normalized without any potassium supplements. In conclusion, patient with resistant hypertension needs to be thoroughly investigated for the secondary causes and the underlying problem needs to be resolved as soon as possible.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML