-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(3): 55-60
doi:10.5923/j.cmd.20140403.03
Anti-Myostatin Reduces Bone Mineral Loss in Ovariectomized Rats
Husam M. Edrees1, 2, Eslam K. Fahmy1, 3
1Department of Physiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
2College of Public Health and Health Informatics, Qassim University, Saudi Arabia
3College of Medicine, Sulaiman Alrajhi Colleges (SRC), Alqassim, Saudi Arabia
Correspondence to: Eslam K. Fahmy, Department of Physiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
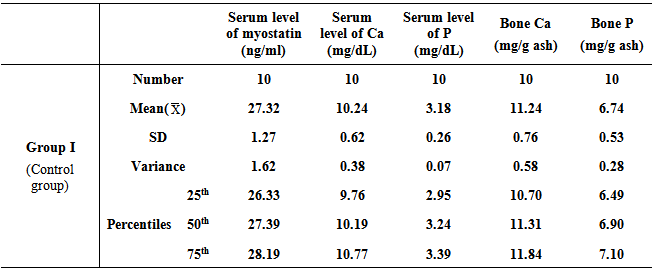
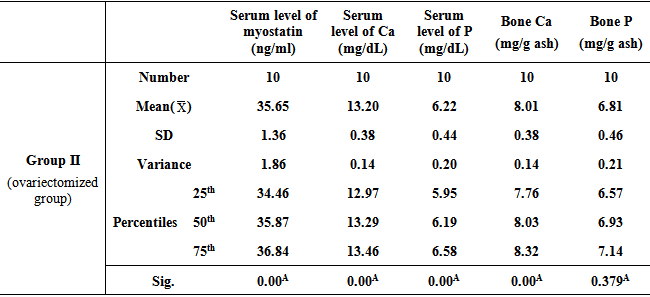
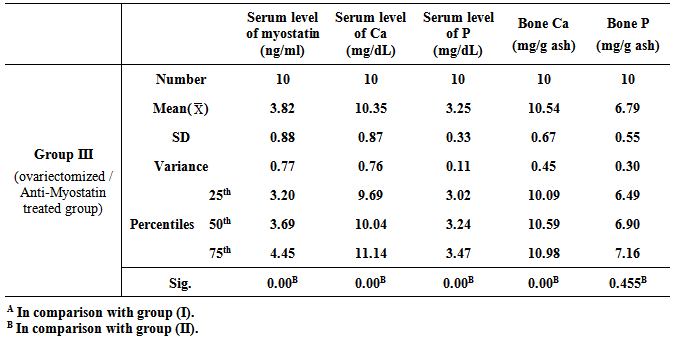
Osteoporosis is a classical age-related disease that affects women as a consequence of estrogen deficiency. Estrogen deficiency has direct as well as indirect impacts on bone metabolism. Studies revealed that in Myostatin-deficient mice, there is an increase in muscle mass which augments increase in bone strength. The aim of this research is to clarify the effect of myostatin blocking on bone mineral density in ovariectomized rats. Design:30 healthy adult female albino rats were used to study the effect of myostatin antibodies (injected subcutaneously at a dose of 5 mg/kg daily for 28 days) against osteoporotic effect of estrogen deficiency induced by bilateral ovariectomy. Osteoporotic effect was assessed by analysis of serum levels of myostatin, calcium, phosphorus and concentrations of calcium and phosphorous in femur bone. Results:The results of this study showed that in group II (ovariectomized group), the mean values of serum myostatin and calcium were found to be significantly increased (P< 0.001) when compared with that of group I (control group) with significant decrease in concentration of calcium in femur bone (P< 0.001). Moreover, In group III (ovariectomized / anti-myostatin treated group), the mean values of serum myostatin and calcium were found to be significantly decreased (P< 0.001) with significant increase in concentration of calcium in femur bone (P< 0.001) when compared with that of group II (ovariectomized group). Concentration of phosphorous in femur bone was found to be non significant (P > 0.05) in all studied groups. Conclusions:It was observed that myostatin blockage protects from ovariectomy induced osteoporosis by retaining bone calcium content.
Keywords: Osteoporosis, Myostatin Antibody, Ovariectomy, Bone Mineral
Cite this paper: Husam M. Edrees, Eslam K. Fahmy, Anti-Myostatin Reduces Bone Mineral Loss in Ovariectomized Rats, Clinical Medicine and Diagnostics, Vol. 4 No. 3, 2014, pp. 55-60. doi: 10.5923/j.cmd.20140403.03.
Article Outline
1. Introduction
- Osteoporosis is one of the major public health problems associated with aging. It is currently defined as decrease of bone mass per bone volume and characterized by compromised bone strength predisposing to an increased risk of fracture. Bone strength is influenced by bone density and bone quality. Bone consists of about 70% minerals mainly as hydroxyapatite, 5–8% water, and the rest as matrix. About [1-2]. Globally, the size of the aging population is increasing rapidly, and as a corollary the prevalence of age-related musculoskeletal disorders such as osteoarthritis, sarcopenia, and osteoporosis is also increasing [3]. A primary contributor to the morbidity and mortality associated with aging is an increased frequency of falls, and falls are often accompanied by bone fractures. Indeed, falls are the primary factor in more than 90% of bone fractures [4]. In many cases bone fractures limit subsequent capacity for normal daily activities, ambulation, and independence, ultimately leading to assisted living situations which can be financially burdensome. The disability following a fall and fracture also contributes directly to an increase in comoribities such as respiratory infections, which in turn contribute to greater overall age-related mortality [5].Osteoporosis is a classical age-related disease that affects women more often than men. The hypothesis that osteoporosis is a consequence of estrogen deficiency, has been proposed as early as 1941 by Albright and colleagues [6]. Collectively, estrogen deficiency has direct as well as indirect impacts on bone metabolism all of which promote osteoclastogenesis. However, the exact mechanisms of estrogen deficiency in postmenopausal women is continuously being unraveled [1]. The most important risk factor for bone loss in midlife women is the menopause. Women lose about 50% of their trabecular bone and 30% of their cortical bone during the course of their lifetime, about half of which is lost during the first 10 yr after the menopause. Approximately 40% of all postmenopausal women will eventually experience fractures [7].Myostatin is most well recognized as a factor that has potent catabolic and anti-anabolic effects on skeletal muscle [8]. Myostatin is a member of the transforming growth factor β (TGF β) superfamily of secreted proteins. Mature myostatin is then secreted from muscle so it can act locally or systemically via the activin type II A and B receptors [9]. It has been argued that muscle strength accounts for a significant proportion of the variance in bone mass observed among adults. Experimental increases in muscle mass and strength are usually produced through vigorous exercise, introducing confounding variables such as increased bone blood flow and changes in the expression of osteogenic factors such as growth hormone [10]. Previous studies on the skeleton of myostatin-deficient mice indicate that these animals show increased BMD and increased bone mass [11, 12].Myostatin function can be inhibited by follistatin, a myostatin antibody, myostatin propeptide, or a soluble decoy myostatin receptor. Inhibitory myostatin antibodies results in increased muscle mass in several disease models, including muscular dystrophy [13] and cancer cachexia [14]. Previous studies found that the prolonged absence of myostatin attenuated age-related decreases in muscle fiber size and satellite cell activation [15]. Myostatin deficiency also appears to improve bone density and bone regeneration. Hence, myostatin inhibitors may not only improve muscle mass and reduce fall risk but may also increase bone strength. These findings are significant from the perspective of fracture prevention with muscular dystrophy in cases with relatively low bone density [16].Ovariectomized animal is a typical experimental model for investigation of postmenopausal osteoporosis due to estrogen deficiency in women. Recently, both ovariectomized mouse and rat are commonly used for the evaluation of bone tissues in the animal model [17].
2. Material and Methods
2.1. Experimental Animal
- The current study was carried on 30 female adult albino rats 12 – 15 weeks old with body weight 200-250 gm, were obtained from the animal house from faculty of veterinary medicine of Zagazig University. Rats were kept in steel wire cages (10/cage) in the physiology research laboratory in faculty of medicine of Zagazig University under hygienic conditions, rats were kept on the normal diet, which consisted of mixed commercial rat laboratory chow and supplied in separate clean containers. Animals had free access to water, kept at room temperature and were maintained on a 12 hr light/dark cycle. The rats were accommodated to laboratory conditions for two weeks before the experiments were going on. Rats were numbered and randomized into the following groups:Group I: (Control group- n = 10): rats were served as a control group; rats were fed standard rat chow that consisted of 25.8% protein, 62.8% carbohydrates and 11.4% fat (total 12.6 kJ/g) [18].Group II: (ovariectomized group- n = 10): rats were fed on normal diet. All the rats were exposed to bilateral ovariectomy and left after surgical operation for 8 weeks to induce osteoporosis as a result of estrogen hormone loss [19].Group III: (ovariectomized / Anti-Myostatin treated group - n = 10): All the rats were exposed to bilateral ovariectomy [19]. Rats were left after surgical operation for 4 weeks. Rats were injected subcutaneously with rat myostatin monoclonal antibodies (Uscn Life Science Inc. - Wuhan, Hubei, PRC) at 5 mg/kg daily for 28 days [19,20].
2.2. Methods
2.2.1. Bilateral Ovariectomy
- Ovariectomy was performed in rats by bilateral incisions at both right and left flanks under ether anesthesia. The periovarian fatty tissue was identified and exteriorized. For prevention of subsequent hemorrhage, the arterial blood vessels were clamped by an artery forceps. Above the clamped area, both ovaries were removed out by cutting using a scalpel. Muscles and skin were stitched separately.
2.2.2. Sampling of Blood
- At the end of the experimental period and after overnight fasting, at 8:00a.m, blood samples (8 ml/rat) were obtained after scarification of rats, and allowed to clot for 2 hours at room temperature before centrifugation for 20 minutes at approximately 500 rpm [21]. The separated serum was stored at -20°C till the time of measurement. Repeated freezing and thawing were avoided.
2.2.3. Bone Mineral Content
- The soft tissues around the right femur bone were removed and the femur was weighed. The femur bone was dried overnight at 100℃. The femur was then incinerated for 12 hrs at 1000℃ in Muffle apparatus to obtain the ash. The ash was weighed, solubilized with 6 N HCL and quantitatively transferred into volumetric flask then completed to 100 ml with 6 N HCL. The solutions were used for analysis of calcium and phosphorus content in bone ash. [22].
2.4. Laboratory Analysis
2.4.1. Estimation of Serum Myostatin Level
- Using a competitive enzyme-linked immunosorbent assay (ELISA) method using myostatin ELISA kits supplied by Uscn Life Science Inc. - Wuhan, Hubei, PRC.
2.4.2. Estimation of Serum Calcium Level
- Concentrations of calcium in serum samples were determined spectrophotometrically using specific diagnostic reagent kits (BioMérieux, France) [23].
2.4.3. Estimation of Serum Phosphorus Level
- Concentrations of phosphorus in serum samples were determined spectrophotometrically using specific diagnostic reagent kits (BioMérieux, France) [24].
2.4.4. Estimation of Bone Calcium Content
- Calcium content in bone ash was determined using atomic absorption spectrophotometer [25].
2.4.5. Estimation of Bone Phosphorus Content
- Inorganic phosphate content in bone ash was determined using spectrophotometer according to the method described by Plummer [26].
2.5. Statistical Analysis
- Results are presented as mean ± standard deviation. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 17.0 (SPSS Inc., Chicago, IL, United States). Repeated measures of analysis of variance (ANOVA) statistical analysis were applied followed by the Student-Newman-Keuls post hoc test. P value <0.05 was considered to be statistically significant.
3. Results
- Table 1, 2, and 3 show serum level of myostatin (ng/ml), serum level of calcium (Ca) (mg/dL), serum level of phosphorous (P) (mg/dL), and concentrations of calcium and phosphorous in femur bone ash in all studied groups expressed as mean ± standard deviation.
|
|
|
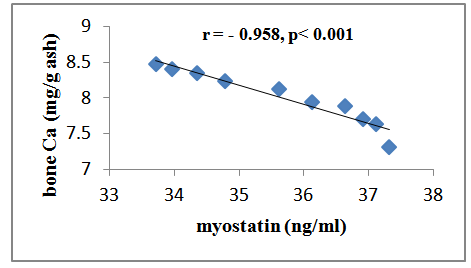 | Figure (1). Correlation between serum myostatin and bone Ca++ in group II |
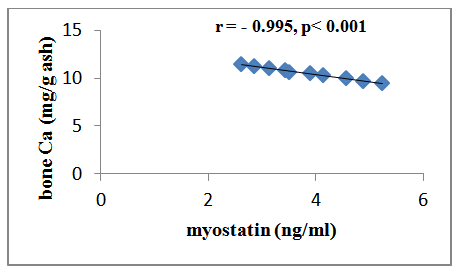 | Figure (2). Correlation between serum myostatin and bone Ca++ in group III |
4. Discussion
- Osteoporosis is characterized by a low bone-mineral density associated with skeletal fractures. The decrease in bone-mineral density is the consequence of an unbalanced bone-remodeling process, with higher bone resorption than bone formation [27]. Myostatin is a member of the bone morphogenetic protein/transforming growth factor-β (TGFβ) super-family of secreted differentiation factors [28]. Myostatin has gained growing attention because of the discovery that myostatin inhibition leads to muscle mass buildup. Myostatin not only plays a key role in muscle homeostasis, but also affects fat and bone [29]. Little is known about the role of myostatin in women in cases of estrogen deficiency as in polycystic ovary syndrome, postmenopause and ovariectomy. A number of strategies were developed to block the effect of myostatin through gene disruption, transgenic expression of myostatin antagonist as it’s propeptide or follistatin or direct administration of an antimyostatin antibody. These strategies were tested on various models of musculoskeletal and metabolic disturbances [30]. In the light of previous data, the present study was designed to clarify the effect of myostatin monoclonal antibodies on bone mineral density in ovariectomized rats. The ovariectomized (OVX) rat model is most commonly used in research on postmenopausal osteoporosis [30].The results of the present study revealed that in group II (ovariectomized group), the mean values were found to be significantly increased for serum myostatin, calcium and phosphorus (P < 0.001, P < 0.001 and P < 0.001 respectively) when compared with that of group I (control group). Concentration of calcium in femur bone ash was found to be significantly decreased (P < 0.001), however, concentration of phosphorous in femur bone ash was found to be non significant (P > 0.05). In agreement with our results, Chen et al. [30] revealed that myostatin level was higher in women with PCOS than those without PCOS, however, their study was a cross-sectional case–control design, and therefore, cannot answer the cause–effect relationship among the androgens, follistatin and myostatin levels. Lots of studies established that after ovariectomy, bone resorption exceeds bone formation initially, causing bone loss [31].In group III (ovariectomized / anti-myostatin treated group), the mean values were found to be significantly decreased for serum myostatin, calcium and phosphorus (P < 0.001, P < 0.001 and P < 0.001 respectively) when compared with that of group II (ovariectomized group). Concentration of calcium in femur bone ash was found to be significantly increased (P < 0.001), however, concentration of phosphorous in femur bone ash was found to be non significant (P > 0.05). Our results regarding treatment with myostatin antibodies are in line with Buehring and Binkley [29] who stated that elevated myostatin levels lead to decreased bone mineral density (BMD) and myostatin inhibition improved BMD and increase muscle and bone mass. It was found that myostatin acts via activation of activin receptor and inhibition of myostatin/activin with activin type IIB receptor immunoglobulin increases bone mineral density and serum levels of markers of bone formation [32], accelerates bone fracture healing in disease models, improves insulin sensitivity, reduces excessive adiposity and attenuates systemic inflammation [33].Interestingly, in contradiction to our results, Arounleut et al. [34] showed that, pharmacological inhibition of myostatin with (propeptide-Fc) increases muscle mass and muscle fiber size in aged mice but does not increase bone density or bone strength. A comparative study between different myostatin blockers conducted by Bialek [28] showed that, myostatin inhibition with either a myostatin neutralizing antibody (Mstn-mAb) or a soluble myostatin decoy receptor (ActRIIB-Fc) increased muscle mass, however, only ActRIIB-Fc increased bone mass in the distal femur and lumbar vertebrae.Moreover, a significant negative correlation was recorded between serum level of myostatin and concentration of calcium in femur bone ash in group II (ovariectomized group) (r = - 0.958; p<0.001) and group III (ovariectomized / anti-myostatin treated group) (r = - 0.995; p<0.001).
5. Conclusions
- The present study suggests that treatment with myostatin blockers can protect against estrogen deficiency induced osteoporosis.
ACKNOWLEDGMENTS
- The authors would like to acknowledge the supporting team of technicians in the Department of Physiology, Zagazig University for their assistance in the experimental work and laboratory analysis. This research received no specific grant from any funding agency in the public commercial or not-for-profit sectors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML